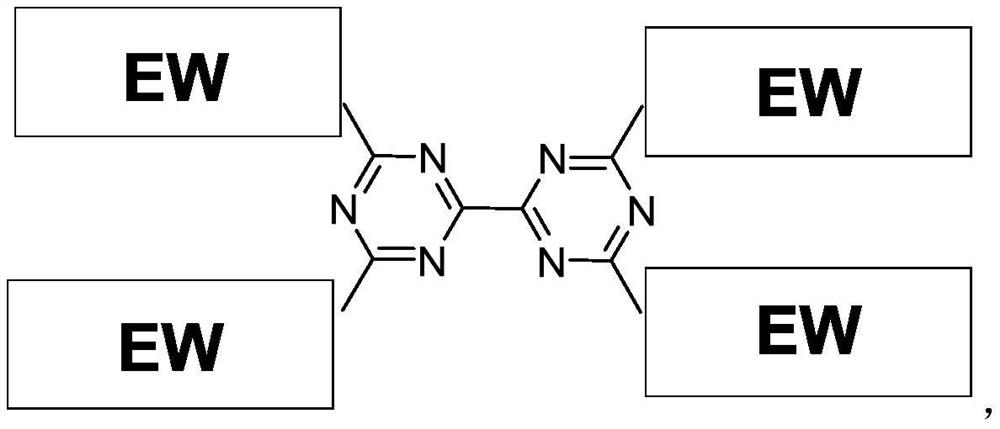
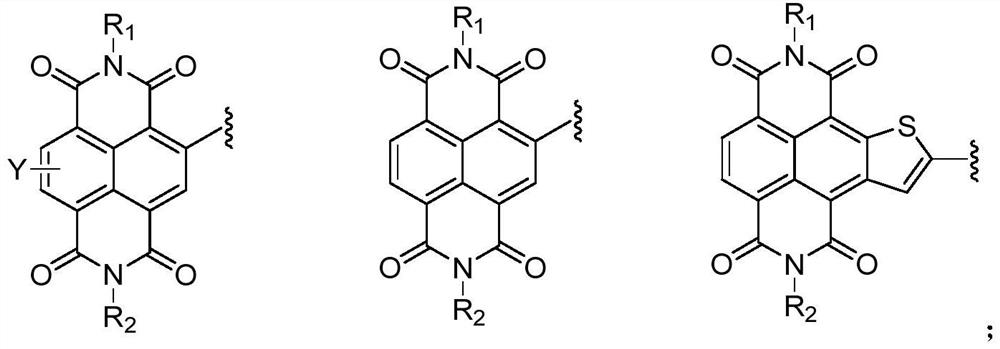
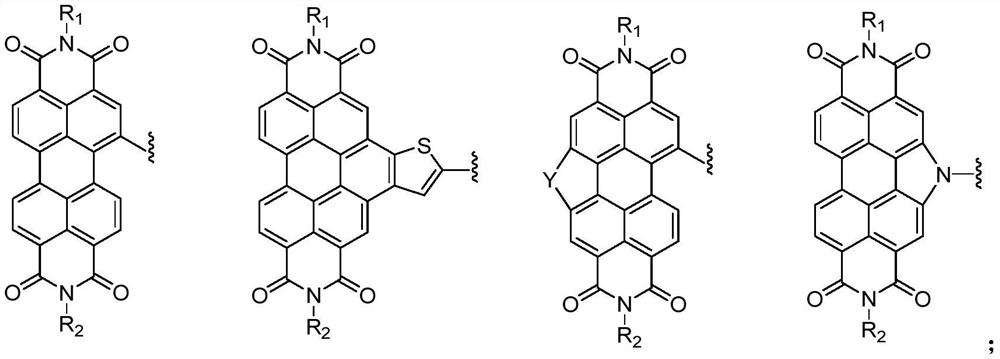
Compound containing triazine group and application of compound as three-dimensional electron acceptor material
A technology of electron acceptor materials and triazine groups, which is applied in the field of three-dimensional electron acceptor materials and compounds containing bitriazine groups, can solve the problems of energy conversion efficiency to be improved and achieve good commercial application prospects , Avoid the effect of too strong aggregation and small space hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A compound containing triazine group, its preparation method is as follows:
[0028] 1) Add compound 1 (2eq), cyanuric chloride (1eq), tetrakistriphenylphosphine palladium catalyst (0.05eq) and cuprous iodide (0.1eq) into the reaction flask, pump out nitrogen, and inject oxygen-free carbonic acid Potassium aqueous solution (2mol / L) and appropriate amount of toluene were reacted overnight at 80°C, the product was washed with water and extracted three times with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and dichloromethane was used as eluent Silica gel column chromatography was carried out to obtain product 2 with a yield of 65%;
[0029] The reaction formula is as follows:
[0030]
[0031] 2) Compound 2 was dissolved in DMF, 2 times the molar amount of reduced copper powder was added, heated to reflux overnight, and the final product 3 was obtained by silica gel column c...
Embodiment 2
[0035] A compound containing triazine group, its preparation method is as follows:
[0036] 1) Add compound 4 (2eq), cyanuric chloride (1eq), tetrakistriphenylphosphine palladium catalyst (0.05eq) and cuprous iodide (0.1eq) into the reaction flask, exhaust nitrogen, inject oxygen-free carbonic acid Potassium aqueous solution (2mol / L) and appropriate amount of toluene were reacted overnight at 80°C, the product was washed with water and extracted three times with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and dichloromethane was used as eluent Silica gel column chromatography was carried out to obtain product 5 with a yield of 75%;
[0037] The reaction formula is as follows:
[0038]
[0039] 2) Compound 5 was dissolved in DMF, 2 times the molar amount of reduced copper powder was added, heated to reflux overnight, and the final product 6 was obtained by silica gel column chroma...
Embodiment 3
[0043] A compound containing triazine group, its preparation method is as follows:
[0044] 1) Add compound 7 (2eq), cyanuric chloride (1eq), tetrakistriphenylphosphine palladium catalyst (0.05eq), cuprous iodide (0.05eq) into the reaction flask, pump out nitrogen, inject anaerobic anaerobic Water toluene and DMF appropriate amount, reflux reaction for 24h, the product was washed with water and extracted three times with dichloromethane, the organic phase was combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and silica gel column chromatography was separated with dichloromethane as eluent , to obtain product 5 with a yield of 75%;
[0045]
[0046] The reaction formula is as follows:
[0047] 2) Compound 8 was dissolved in DMF, 2 times the molar amount of reduced copper powder was added, heated to reflux overnight, and the final product 9 was obtained by silica gel column chromatography with a yield of 80%.
[0048] The reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com