Preparation method of nitrogen-sulfur co-doped carbon-coated transition metal nano sulfide electrochemical oxygen catalyst
A nitrogen-sulfur co-doping, transition metal technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of scarcity and high price hindering the large-scale practical application of batteries, and achieve The effects of abundant reserves, good conductivity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
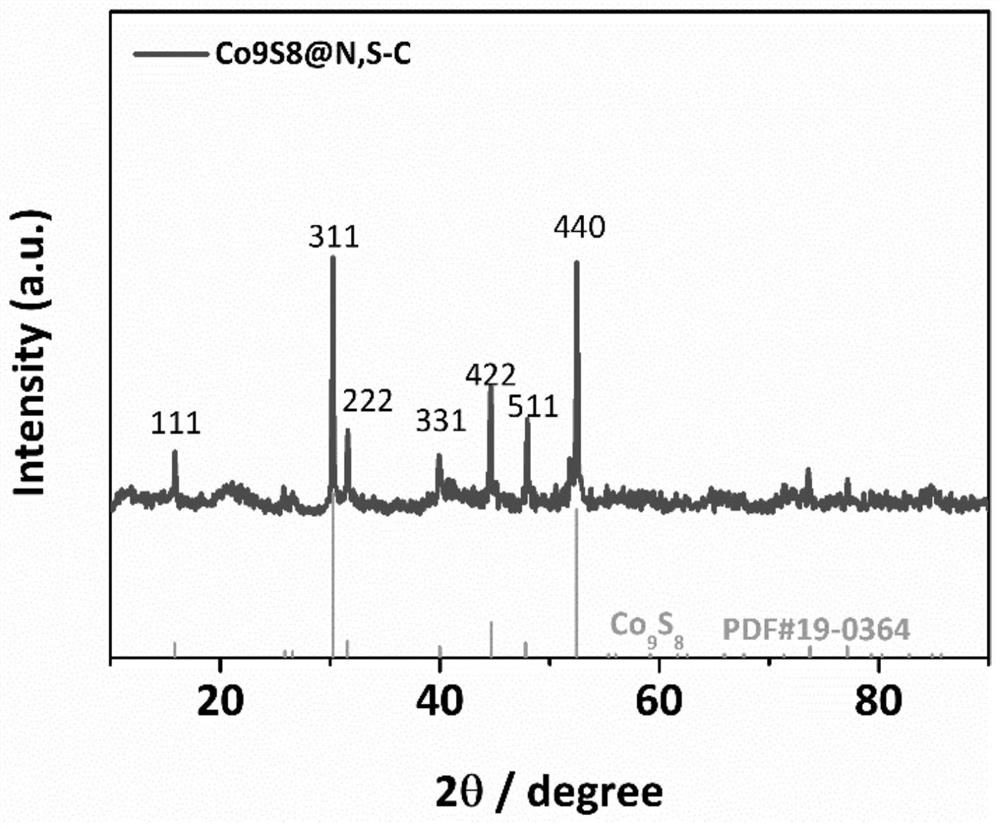
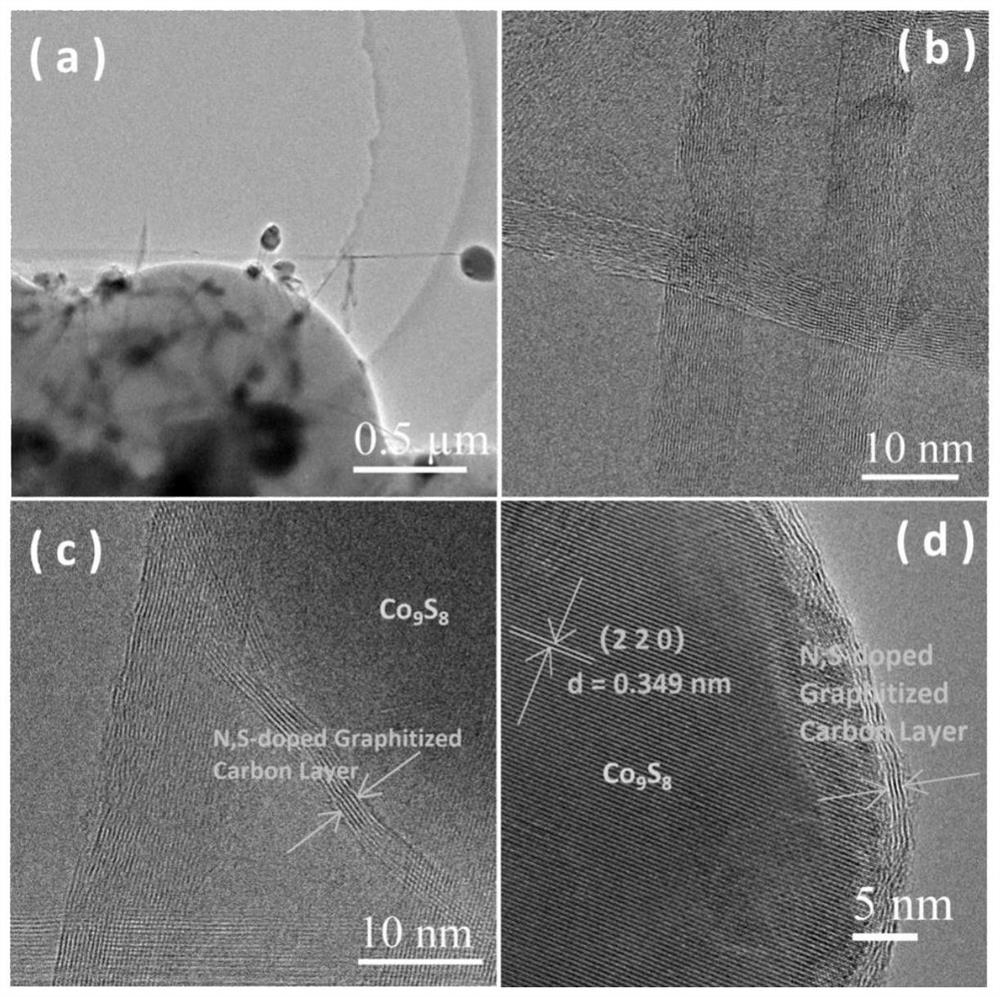
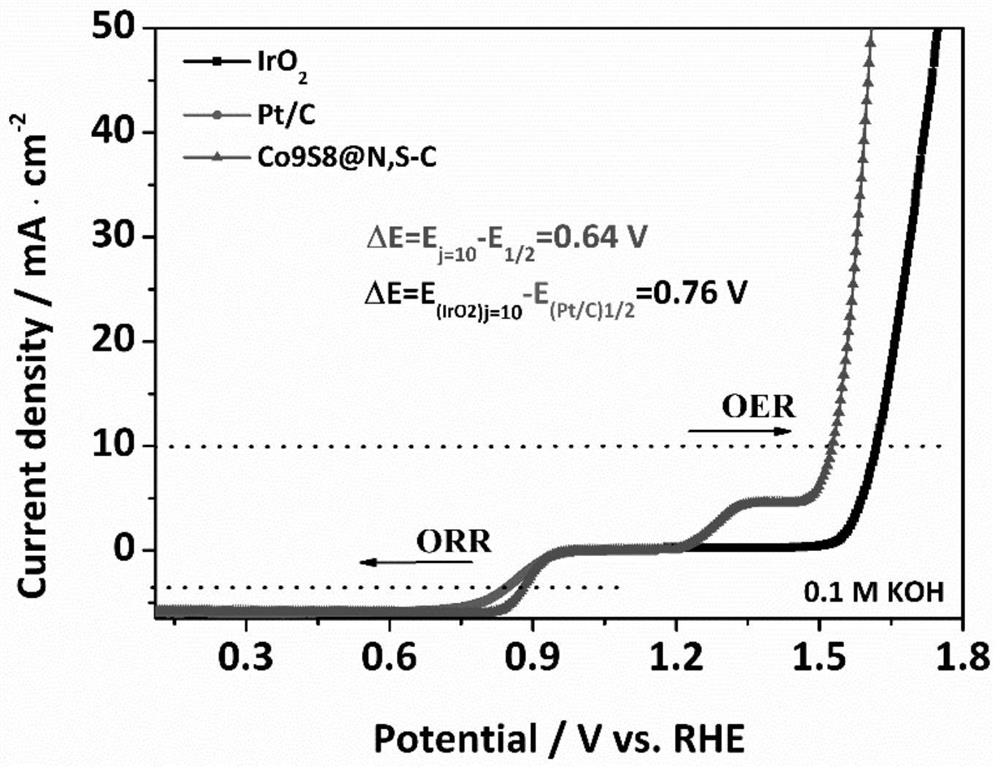
Image
Examples
Embodiment 1
[0030] A method for preparing a nitrogen-doped carbon coated transition metal nanosulfide electrochemical oxygen catalyst, the specific operation steps are as follows:
[0031] (1) 2,6-diacetylpyridine 1.3 g was placed in 150 mL of flat bottom flask, and 50 mL of anhydrous ethanol was added to the flat bottom flask, stirred until 2,6-diacetylpyridine completely dissolved, according to acetyl And the molar ratio of the amino group was 1: 1 to 1.99 g of 2,2'-di aminoisulfide sulfonthionic ether, stirred to completely dissolve, add 0.1 g of ohoxic acid to the flat flask, stirred for 30 min, and turn the flask to the oil bath. The temperature rose to 60 ° C, reacted at a constant temperature stirring for 10 hours;
[0032](2) After the reaction is cooled to room temperature, then 1.90 g of hexahydrate chloride is added to the room temperature, and the reaction is added to 12 hours while stirring, and the obtained substance after the reaction is removed by a rotary evaporator to evapor...
Embodiment 2
[0036] A method for preparing a nitrogen-doped carbon coated transition metal nanosulfide electrochemical oxygen catalyst, the specific operation steps are as follows:
[0037] (1) 2,6-diacetylpyridine 1.3 g was placed in 150 mL of flat flask, and 50 ml of organic solvent anhydrous ethanol was added to the flat flask, stirred until 2,6-diacetylpyridine completely dissolved, press The acetyl and the molar ratio of the amino group was added to 1.98 g of the sulfur-containing amino monomer 4,4-di-diiminary thion ether, stirred to completely dissolve, add 0.1 g of ohoxic acid to the flat flask, stirred for 30min, and turn the flask In the oil bath, heated to 80 ° C, reacted at a constant temperature stirring for 12 hours;
[0038] (2) After the step (1), the resulting solution was cooled to room temperature, and 2.16 g of the transition metal-containing inorganic salt hexahydrachloride was added while stirring, 10 hours of reaction at room temperature, and the obtained substance was r...
Embodiment 3
[0043] A method for preparing a nitrogen-doped carbon coated transition metal nanosulfide electrochemical oxygen catalyst, the specific operation steps are as follows:
[0044] (1) 2,6-diacetylpyridine 1.0 g was placed in three 150 ml flat flasks, and 50 ml of organic solvent anhydrous ethanol was added to the flat bottom flask, stirred until 2,6-diacetylpyridine is completely The molar ratio of the acetyl group and the amino group was added to 1.31 g of the sulfur-containing amino monomer 2,2'-di-diiminated aminogenated 1.31 g, stirred to completely dissolve, and 0.1 g of ohoxic acid was added to the flat bottom flask, stirred for 30min , Turn the flask to the oil bath, warmed to 100 ° C, reacted at a constant temperature stirred for 8 hours;
[0045] (2) After the reaction is cooled to room temperature after reacting step (1), 3.6 g, 1.2 g, 0.4 g of a transition metal hexahydride-containing nickel-containing nickel-containing nickel-containing nickel-containing nickel-containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com