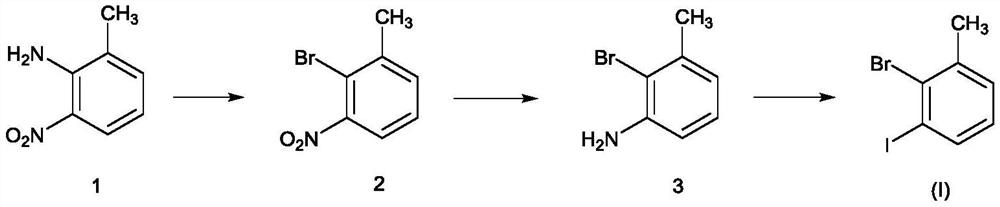
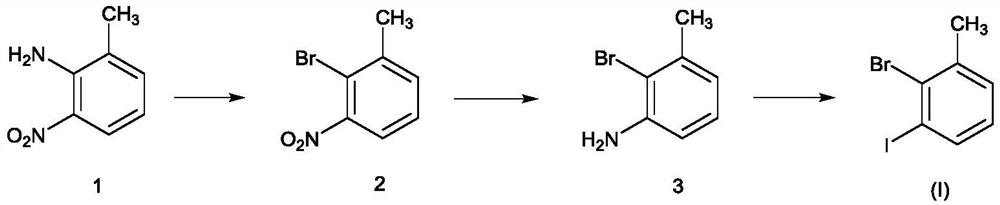
Novel method for preparing 3-iodo-2-bromotoluene
A novel technology of bromotoluene, which is applied in the field of preparing 3-iodo-2 bromotoluene, can solve problems such as difficult centrifugation, troublesome post-reduction treatment, and potential safety hazards, and achieves wide and easy-to-obtain raw material sources, simple reagents, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 2-amino-3-nitrotoluene (21.8kg), water (130.9kg), and dioxane (67.4kg) into the reaction kettle, and the brown turbid liquid was heated to reflux, and 40% hydrogen was added dropwise under reflux Bromic acid (97.1kg), dropwise. Keep warm for 2 hours, cool down, cool down to 0-20°C, control at 0-20°C, add sodium nitrite solution (9.9kg / 87.3kg) dropwise, after dropping, keep warm for 2 hours, and configure potassium bromide, water, 40% at the same time Hydrobromic acid solution (23.7kg / 130.9kg / 97.1kg), lowered to 0-20°C for later use. Add dropwise to potassium bromide, water, in the system of 40% hydrobromic acid solution (a large amount of foams are generated in the process) after the insulation is completed. After the dropwise addition, keep warm at 0-5°C for 30-60 minutes, raise the temperature to 60°C for 30-60 minutes, and cool down to room temperature overnight. After TLC detects that there is no reactant, the reaction is complete. An appropriate amount of et...
Embodiment 2
[0034] Add 2-bromo-3-nitrotoluene (20.3kg), ethanol (203.0kg), and 5% palladium carbon (0.2kg) into the hydrogenation kettle. After the replacement is completed, control the pressure to 0.2-0.6MPa and the temperature to 45-55°C , hydrogenation reduction, sampling point plate detection, bromide disappears, hydrogenation is completed, cooled to room temperature, discharged, filtered, and desolvated under reduced pressure. A brown oil was obtained. Concentration under reduced pressure was carried out, and the temperature was controlled to be below 100° C. and evaporated to obtain 15.7 kg of pure product with a molar yield of 90%.
Embodiment 3
[0036] Add 2-bromo-3-aminotoluene (8.8kg), dioxane (26.2kg), and water (53.9kg) to reflux, add concentrated sulfuric acid (18.9kg) dropwise, and reflux for 1 to 2 hours. After reflux, cool down to 0-20°C and start adding sodium nitrite aqueous solution (3.3kg / 35.1kg) dropwise, and control the temperature between 0-20°C for about 1 hour. After dropping, stir at 0-20°C for 2 hours, and prepare potassium iodide aqueous solution (9.0 / 35.1 / kg). Concentrated sulfuric acid (18.9kg), after addition, was cooled to 0°C for use. Add the system dropwise to the reaction kettle with potassium iodide and concentrated sulfuric acid solution (control the temperature to 0-20°C, after about 1 hour, there will be more foam and more elemental iodine will be generated). After dropping, control the temperature at 0-20°C and stir for 2 hours, raise the temperature to 60°C for 2 hours, and cool down to room temperature for 2 hours (or overnight). The TLC raw material disappeared, and ethyl acetate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com