Method for preparing 1, 2-benzisothiazolin-3-one through catalytic oxidation
A benzisothiazoline, catalytic oxidation technology, applied in chemical instruments and methods, catalytic reactions, physical/chemical process catalysts, etc. The effect of less product, less waste, simple and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
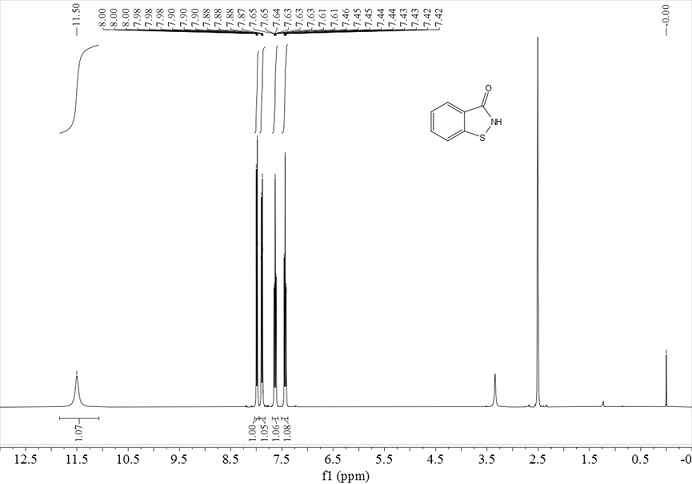
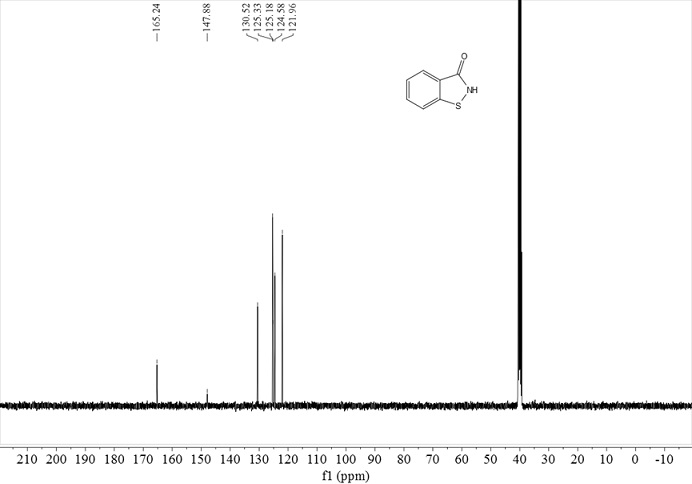
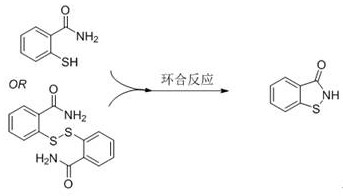
[0028] In a 250 mL reactor, put 15.28 g of a mixture of 2-mercaptobenzamide and 2,2’-dithiodibenzamide (molar ratio 3:1), 0.42 g of Mn(OH) 3 , 0.60 g of triethanolamine and 160 mL of ethanol; heated to 120°C with stirring, fed oxygen to keep the pressure in the reactor at 0.2 MPa, stopped the reaction after 10 hours of reaction, removed ethanol by rotary evaporation, added 100 mL of water and stirred for 20 min , filtered, and the filter cake was dried to obtain 12.23 g of white solid BIT with a yield of 90%, a melting point of 154-156°C, and a purity of 98% according to liquid chromatography analysis.
Embodiment 2
[0030] In a 250 mL reactor, put 30.6 g of 2-mercaptobenzamide, 0.11 g of manganese carbonate monohydrate, 0.28 g of 2-dimethylaminoethylamine, 100 mL of water and 50 mL of methanol; heat to 40 ℃, press air, keep the pressure in the reactor at 1.0 MPa, stop the reaction after 40 hours of reaction, remove methanol by rotary evaporation, cool, filter, wash with water, and dry to obtain 29.6 g of BIT with a yield of 98%. Chromatography analysis product purity is 99%.
Embodiment 3
[0032] In a 250 mL reactor, put 45.95 g 2,2'-dithiodibenzamide, 0.02 g 2,2'-bipyridyl manganese (II) complex and 130 mL DMF; heat to 80 ℃, feed oxygen, keep the pressure in the reactor at 0.4 MPa, stop the reaction after 7 hours of reaction, distill off most of the DMF under reduced pressure, add 100 mL of water and stir for 40 min, filter, and dry the filter cake to obtain 44.85 g of white solid BIT , the yield was 99%, the melting point was 154-156°C, and the purity of the product by liquid chromatography analysis was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com