A kind of indole compound and its preparation method and application
A technology of compounds and indoles, applied in the field of medicinal chemistry, can solve the problem of few ligands, and achieve the effect of strong binding constant, high fluorescence intensity and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
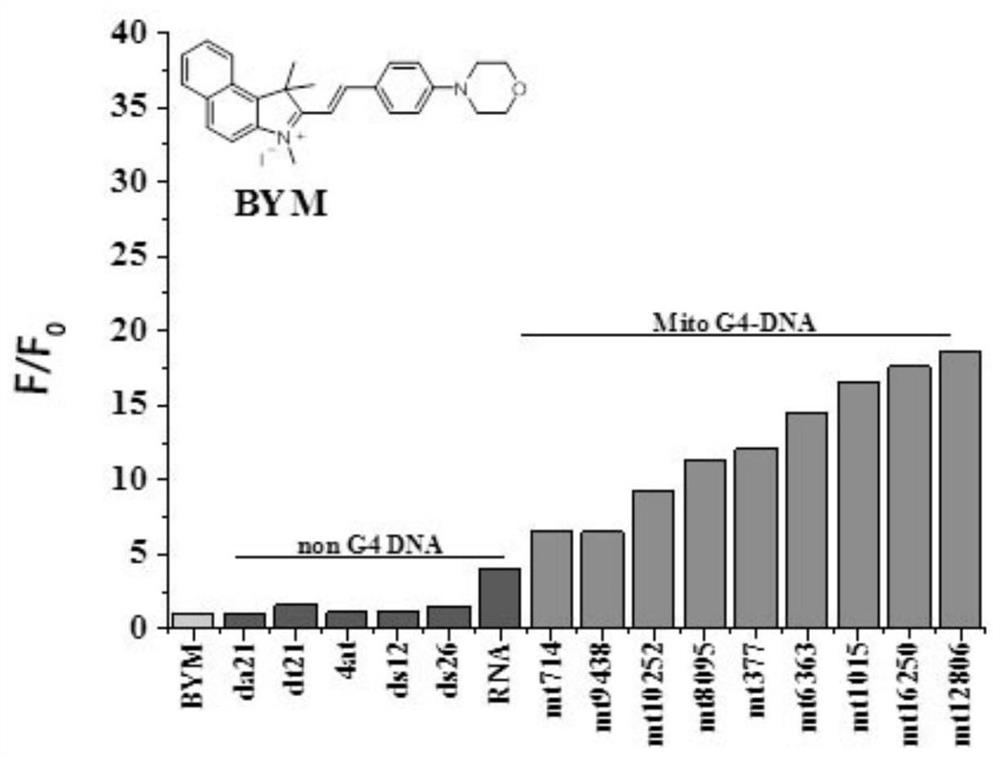
[0058] The preparation of the indole compound BYM described in this example comprises the following steps:
[0059] (1) Weigh 0.4g (1.912mM) of 1,1,2-trimethyl-1H-benzo[e]indole in an explosion-proof bottle, 4mL (42mM) sulfolane as a solvent, and mix the two with ultrasound Evenly, add 1,1,2-trimethyl-1H-benzo[e]indole twice the molar amount (3.824mM) of methyl iodide under fume hood conditions, place the reaction system in an oil bath and open Magnetic stirring, the reaction temperature is 60°C, and the reaction time is 24 hours; after the reaction system is cooled to room temperature, add a small amount of ethyl acetate to fully shake, let it stand for a while, precipitate crystals, vacuum filter, rinse the filter cake with ethyl acetate, and dry Obtain light yellow crystal intermediate A 1 0.58g, thin plate chromatography shows no by-product, intermediate A 1 The crude yield is 86%;
[0060] The intermediate A 1 The synthetic route of is as follows:
[0061]
[006...
Embodiment 2
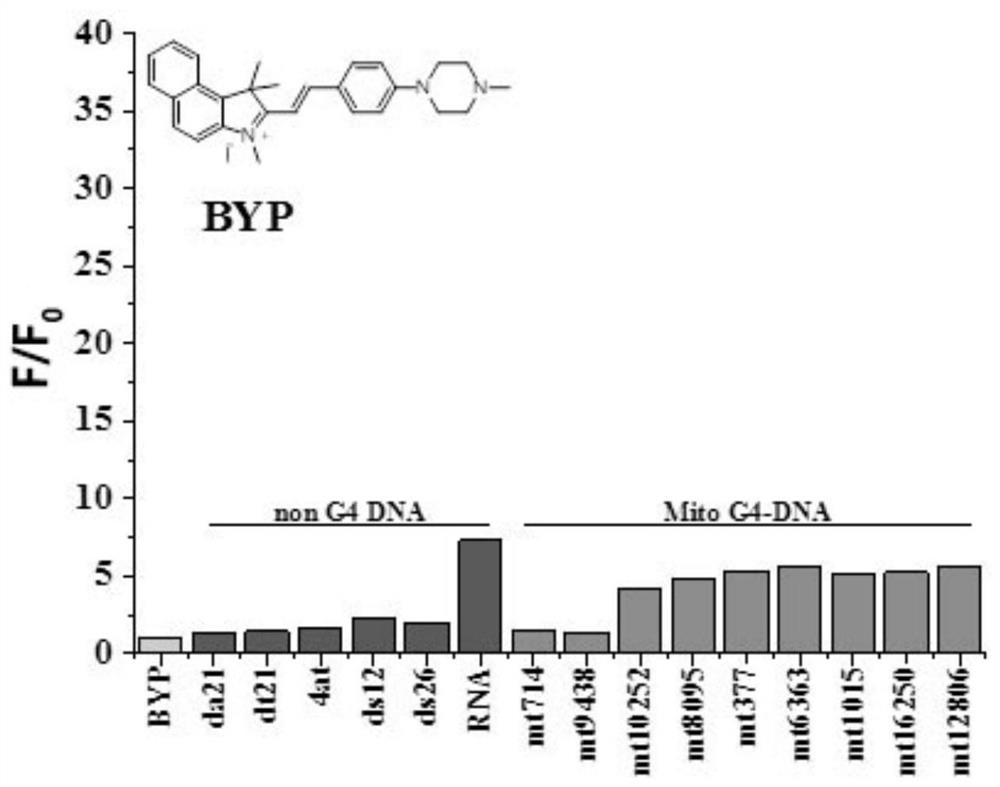
[0068] The preparation of the indole compound BYP described in this example comprises the following steps:
[0069] (1) the intermediate A 1 The preparation method is identical with embodiment 1;
[0070] (2) Weigh 0.2g (0.569mM) of Intermediate A 1 and 0.140mg (0.683mM) 4-(4-methylpiperazine) benzaldehyde was placed in an explosion-proof bottle, 3mL (34.25mM) ethanol was used as a solvent, and ultrasonication was used for a while to make the mixture uniform, and the reaction system was placed in an oil bath and Turn on the magnetic stirring, the reaction temperature is 60°C, and the reaction time is 12 hours; after the reaction system is cooled to room temperature, solids will precipitate out, and the dark pink powder is obtained by suction filtration, and then the components in the red powder are separated by column chromatography , finally obtained 0.240 g of indole compounds, counted as BYP, and the yield was 35%. The indole compound BYP is a dark red crystal. The hydr...
Embodiment 3
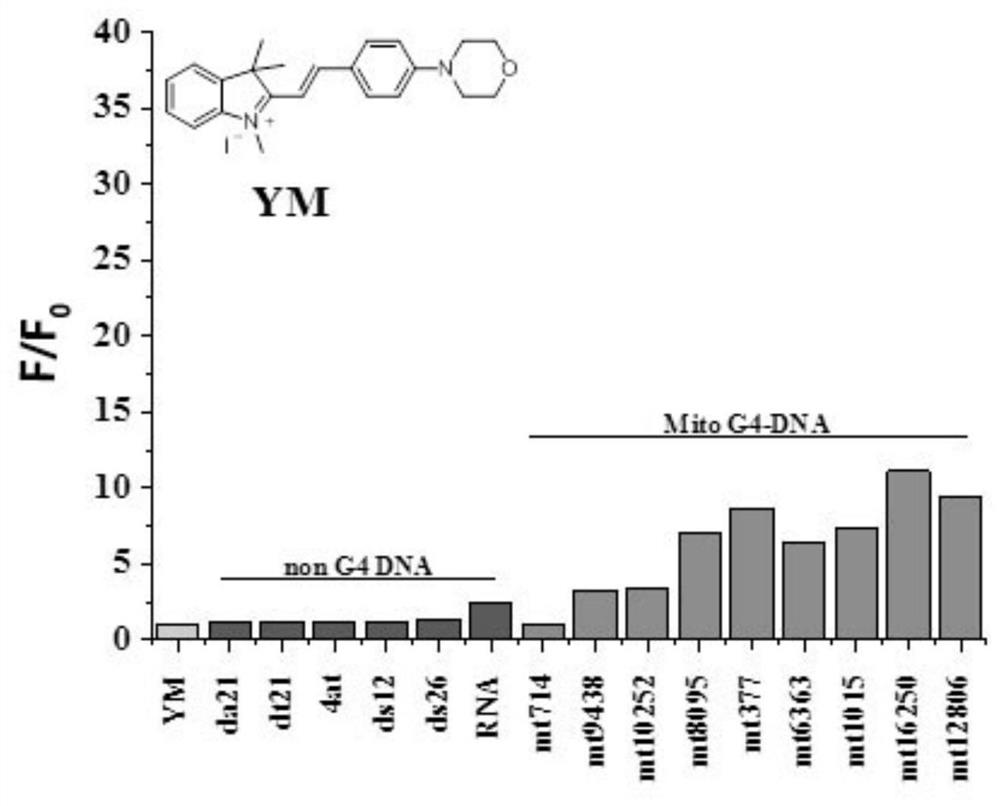
[0076] The preparation of the indole compound YM described in this embodiment comprises the following steps:
[0077](1) Weigh 0.4g (2.480mM) of 2,3,3-trimethyl-3H-indole into an explosion-proof bottle, 4mL (42mM) sulfolane as a solvent, ultrasonically mix the two evenly for a while, ventilate Add twice the molar amount (4.96mM) of methyl iodide under hood conditions, place the reaction system in an oil bath and turn on magnetic stirring, the reaction temperature is 60°C, and the reaction time is 24h; after the reaction system is cooled to room temperature, add Ethyl acetate was fully shaken, stood still for a while, crystals were precipitated, vacuum filtered, and the filter cake was rinsed with ethyl acetate, dried to obtain light pink intermediate A 2 Crystal 0.52g, thin plate chromatography showed no by-products, intermediate A 2 The crude yield is 70%;
[0078] The intermediate A 2 The synthetic route of is as follows:
[0079]
[0080] (2) Weigh 0.2g (0.664mM) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com