Inhibitor of metallo-beta-lactamase produced by multi-drug resistant bacteria and preparation method thereof
A technology of inhibitors and lactams, applied in antibacterial drugs, pharmaceutical formulations, chemical instruments and methods, etc., can solve problems such as the inhibition of class B MBL that has not yet been proven, and achieve the effect of prolonging or expanding time and scope and preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] virtual library screening
[0063] To find novel compounds that inhibit NDM-1, the most clinically important MBL, a structure-based virtual screening was performed using ICM-VLS software. The crystal structure of NDM-1 (PDB ID, 3SPU) was analyzed using the PocketFinder algorithm available in the Internal Coordinate Mechanics (ICM) software program (Molsoft, LLC) (King et al., Protein Science, 20, 1484-1491 (2011)).
[0064] Using the ICM force field and the RMS (root mean square) gradient is The distance-dependent dielectric potential of , to prepare protein structures by three-dimensional (3D) protonation, water molecule deletion, and energy minimization.
[0065] Define the ligand binding site as around the active site cleft and selected it as the target site for virtual library screening (VLS) using ICM-VLS software (Molsoft, LLC). ICM-VLS uses global optimization with biased probabilistic Monte Carlo conformational search to rapidly dock a fully flexible all-at...
Embodiment 2
[0074] in vivo screening
[0075] To discover the enhanced activity of combinations of imipenem (fixed 8 μg / mL) and chemicals, 6,600 compounds of our chemical library were tested in 96-well plates at 200 μM against bla NDM-1 Initial screening for Klebsiella pneumoniae. Briefly, we cultured 1 × 10 7 cells / mL and dispense 10 μL of the solution into a 96-well plate using a multichannel pipette. We incubated the 96-well plate at 37 °C for 24 h. Then, we use OD 600 Cell viability was determined by the absorbance at 100 Å, and compounds were selected that reduced the turbidity of the cultures by more than 60% relative to untreated controls. For carrying bla NDM-1 An initial screen of 6,600 compounds in Klebsiella pneumoniae yielded four "hits" that reduced cell viability to below 60 percent.
[0076] Four compounds (OCL-1, OCL-2, OCL-3 and OCL-4) were tested in a checkerboard assay to determine their individual and combined efficacy (Table 2). Selected compound combinations w...
Embodiment 3
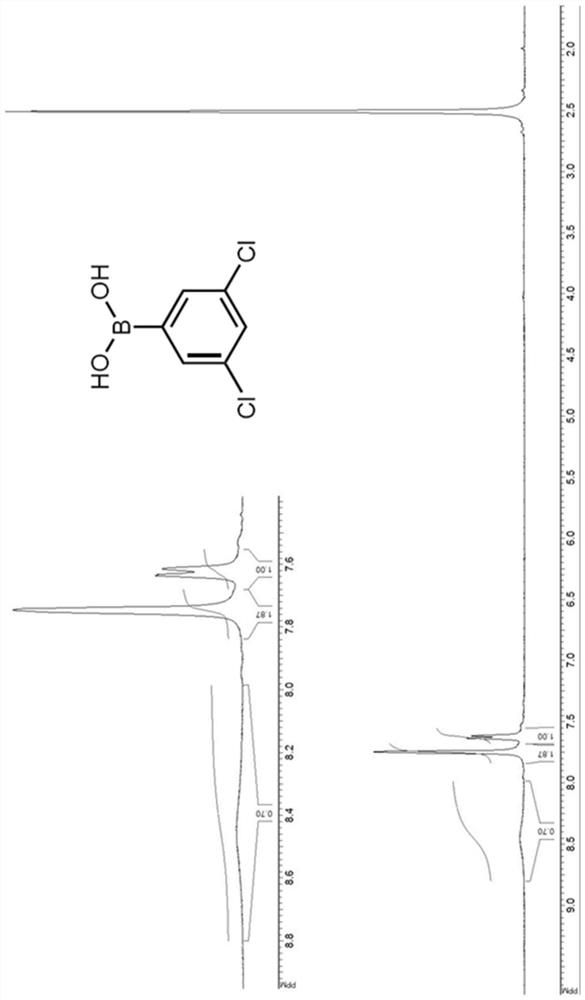
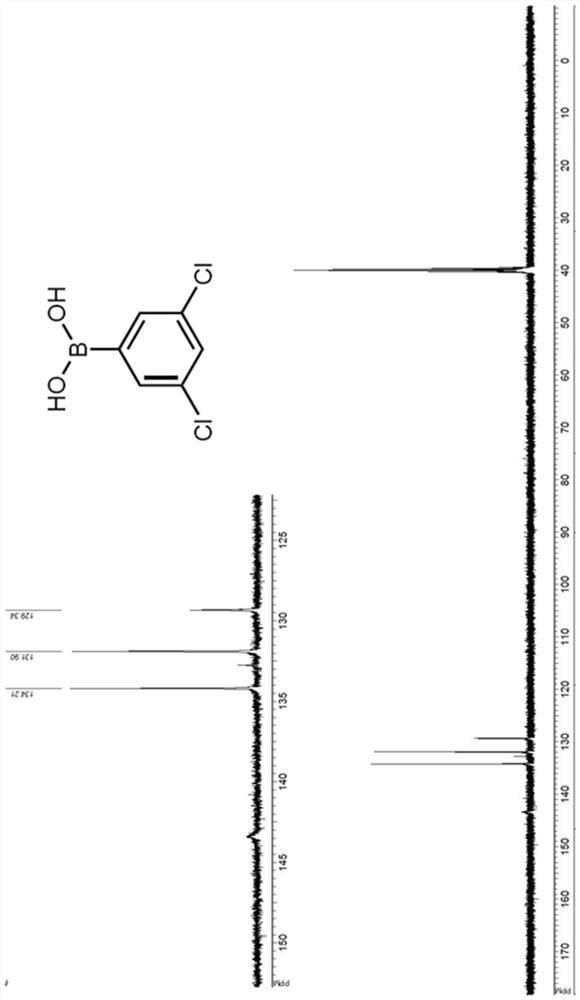
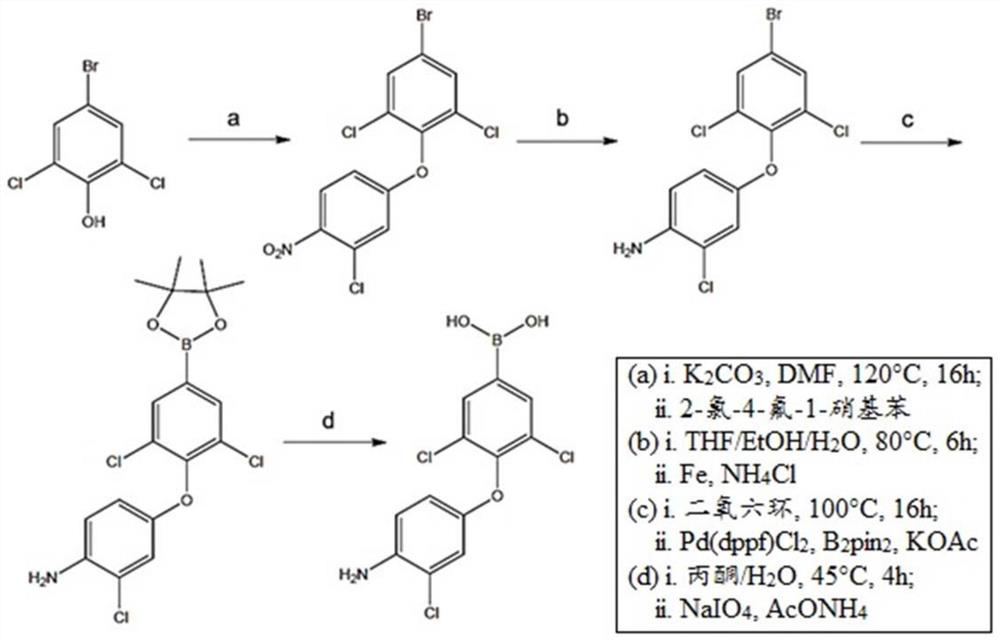
[0080] Synthesis and Characterization of Novel Compounds
[0081] Organometallic reactions were performed in oven-dried glassware under an argon atmosphere and using anhydrous solvents. Anhydrous tetrahydrofuran (THF) and diethyl ether were obtained by standard methods and THF was freshly distilled from sodium benzophenone ketyl under argon before use. All starting chemicals and reagents are commercially available. Chromatographic purification of the compounds was performed on silica gel (particle size 0.05-0.20 mm). In Bruker Avance400 (400MHz for 1 H and 125MHz for 13 C) or Bruker Avance500 (500MHz for 1 H and 125MHz for 13 C) Methanol (CD) on a spectrometer 3 OD) recorded in solution 1 H-NMR and 13 C-NMR spectrum. Chemical shifts (δ) are reported in ppm downfield from tetramethylsilane (TMS) as internal standard (s singlet, d doublet, t triplet, m multiplet, br s broad signal). Coupling constants (J) are given in Hz. The purity of the resulting compounds was chec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com