An anthranilamide compound based on entinotine skeleton and its preparation and application
A technology of aminobenzamide and entinostat, which is applied in the field of medicine and can solve problems such as neutropenia and leukopenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
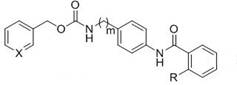
[0026] pyridin-3-ylmethyl 4-(2-aminobenzamido)benzylcarbamate (compound X5, formula II, m=1, X=N)
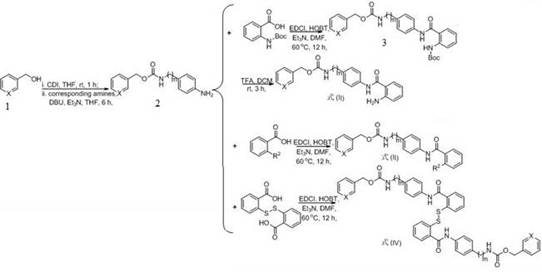
[0027] (1) Add 3-pyridinemethanol (Formula 6, X=N, 10 mmol), N,N'-carbonyldiimidazole (CDI, 11 mmol) and tetrahydrofuran (50 mL) to a 100 mL reaction flask, and react at room temperature for 1 h. 4-Aminobenzylamine (11 mmol), 1,8-diazabicycloundec-7-ene (DBU, 10 mmol) and triethylamine (15 mmol) were added and reacted at room temperature for 6 h. After the completion of the reaction monitored by TLC, the solvent was evaporated by rotary evaporation, and the product pyridin-3-ylmethyl 4-aminobenzylcarbamate (formula 7, m=1, X=N) was obtained by column chromatography in a yield of 90%.
[0028] (2) pyridin-3-ylmethyl 4-aminobenzylcarbamate (1 mmol), 2-((tert-butoxycarbonyl)amino)benzoic acid (1.2 mmol) 1-ethyl-(3-dimethylaminopropyl)carbonyl Diimine hydrochloride (EDCl, 1.2 mmol), 1-hydroxybenzotriazole (HOBt, 1.3 mmol) were placed in a 50 ml round bottom flask and replaced with...
Embodiment 2
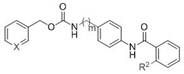
[0031] pyridin-3-ylmethyl 4-(2-(methylamino)benzamido)benzylcarbamate (compound X6, formula II, m=1, X=N, R 2 =NHCH 3 )
[0032] (1) Add 3-pyridinemethanol (Formula 6, X=N, 10 mmol), N,N'-carbonyldiimidazole (CDI, 11 mmol) and tetrahydrofuran (50 mL) to a 100 mL reaction flask, and react at room temperature for 1 h. 4-Aminobenzylamine (11 mmol), 1,8-diazabicycloundec-7-ene (DBU, 10 mmol) and triethylamine (15 mmol) were added and reacted at room temperature for 6 h. After the completion of the reaction monitored by TLC, the solvent was evaporated by rotary evaporation, and the product pyridin-3-ylmethyl 4-aminobenzylcarbamate (formula 7, m=1, X=N) was obtained by column chromatography in a yield of 90%.
[0033] (2) pyridin-3-ylmethyl 4-aminobenzylcarbamate (1 mmol), N-methylanthracene (1.2 mmol), 1-ethyl-(3-dimethylaminopropyl) carbamide hydrochloride (EDCl, 1.2 mmol), 1-hydroxybenzotriazole (HOBt, 1.3 mmol) was placed in a 50 ml round bottom flask and replaced with argon...
Embodiment 3
[0035] pyridin-3-ylmethyl 4-(2-(dimethylamino)benzamido)benzylcarbamate (compound X7, formula II, m=1, X=N, R 2 =N(CH 3 ) 2 )
[0036] The raw material N-methylanthracene in step (2) of Example 2 was replaced with 2-(dimethylamino)benzoic acid, and the remaining steps were prepared in the same manner as in Example 2, and the yield was 55%. 1 H NMR (300 MHz, Acetone- d 6 ) δ 12.04 (s, 1H), 8.64 (s, 1H), 8.54 (d, J = 3.8 Hz, 1H), 8.10 (dd, J = 7.8, 1.7 Hz, 1H), 7.82 (d, J =7.8 Hz, 1H), 7.74 (d, J = 8.5 Hz, 2H), 7.56 – 7.48 (m, 1H), 7.46 – 7.35 (m, 2H), 7.31 (d, J = 8.4 Hz, 2H), 7.27 – 7.20 (m, 1H), 6.96 (s, 1H), 5.16 (s, 2H), 4.33 (d, J = 6.2 Hz, 2H), 2.85 (s, 6H). 13 C NMR (75 MHz, Acetone- d 6 ) δ164.82, 157.14, 153.29, 149.85, 149.66, 139.17, 136.70, 135.61, 134.11,133.09, 131.80, 128.79, 128.72, 124.89, 124.38, 121.20, 120.52, 120.42,64.25, 45.28, 44.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com