Low-toxicity antiarrhythmic medicine and preparation method thereof
A low-toxicity, pharmaceutical technology, applied in the direction of drug combination, pharmaceutical formula, organic chemical method, etc., can solve the problem of unclear component conversion, cardiotoxicity and neurotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
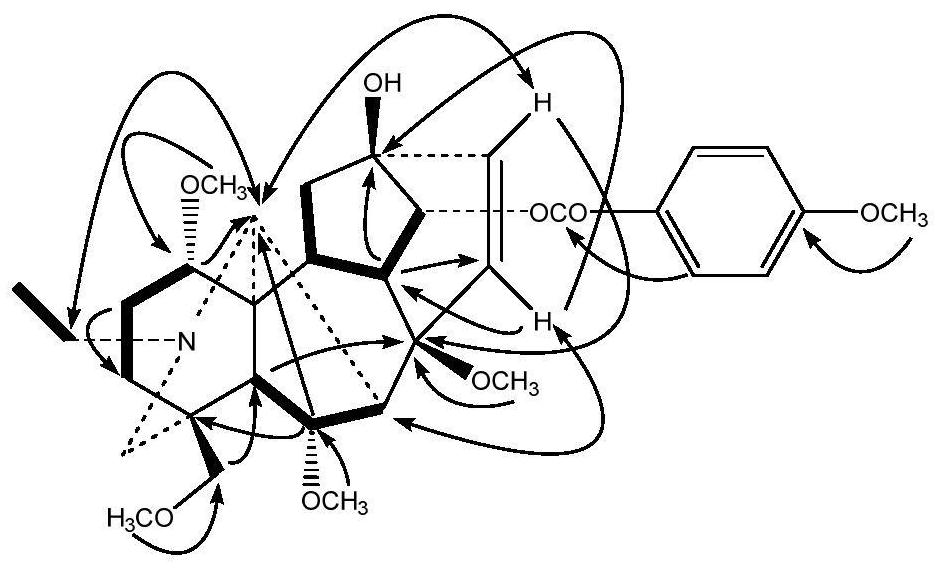
[0048] The preparation of embodiment 1 compound 2 of the present invention
[0049] 1. Preparation of Samples for Column Chromatography Separation
[0050] Take 1.83g of aconitin, put it in a 250mL round-bottomed flask, add 25mL of dichloromethane to dissolve, and evaporate the solvent to dryness at 40°C with a rotary evaporator, so that aconitin is evenly attached to the inner wall of the round-bottomed flask, and Put it in an oil bath at 160°C for 30 minutes, take it out, and let it cool to obtain the processed product of aconitin (1.65 g), which is used for column loading.
[0051] 2. Separation and purification
[0052] After dissolving the processed product of aconitin in dichloromethane, it was separated and purified by silica gel column chromatography (200-300 mesh, 120g), and was respectively purified with petroleum ether-acetone-triethylamine 8:1:0.01 (0.4L), 6:1:0.01 (3L), 3:1:0.01 (1.2L) gradient elution, TLC plate inspection, after combining similar fractions, th...
experiment example 1
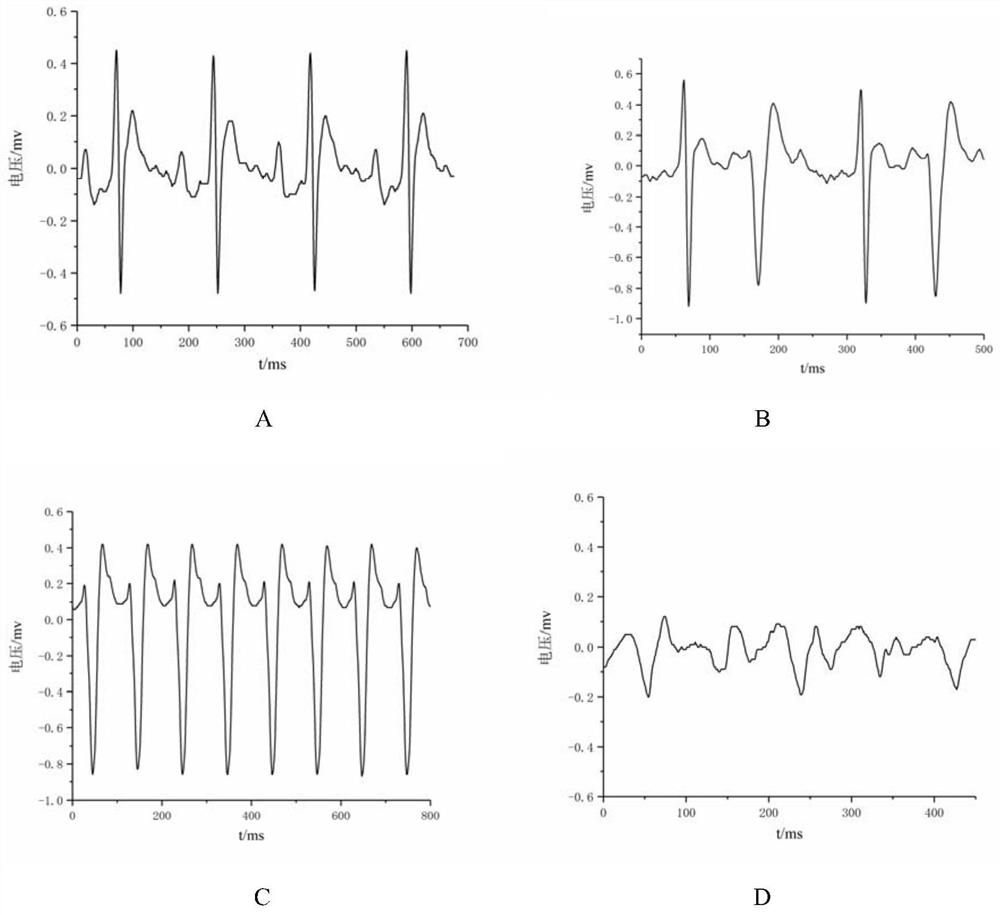
[0065] Cardiotoxicity Test of Experimental Example 1 Aconitin and its Stir-fried Product Compound 2
[0066] 1. Test method
[0067] Take 20 SD rats of SPF grade, half male and half male, and randomly divide them into aconitin group and compound 2 group (n=10, fasting without food and water for 12 hours before the experiment). Anesthetized by intraperitoneal injection of 20% urethane (dose 1.2g / kg), fixed in the supine position, inserted the needle electrode into the subcutaneous of the extremities, and observed the electrocardiogram of lead II of the rat with a BL-420F multifunctional physiological recorder for 20 minutes. ECG changes within 30 minutes after taking the medicine. Through preliminary preliminary experiments, it was found that 0.10mg / kg of aconitin can cause ventricular premature beat (VPB), ventricular tachycardia (VT) and ventricular fibrillation (VF) in rats, etc. Arrhythmia, by observing whether compound 2 causes the above arrhythmia at the same dose, it c...
experiment example 2
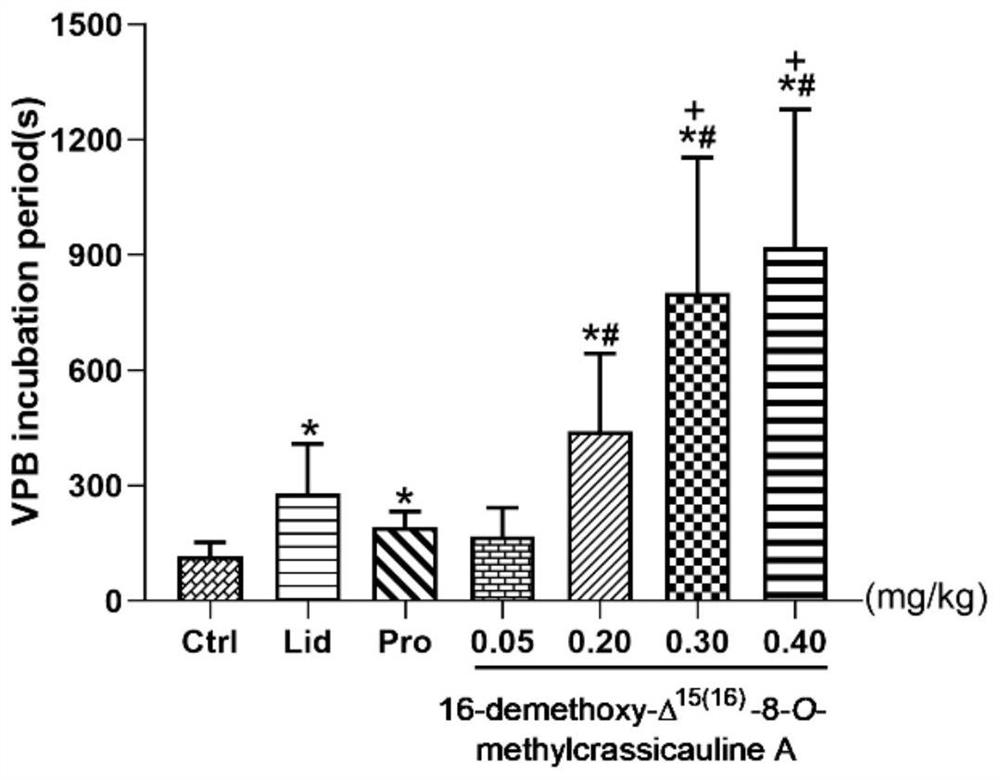
[0074] Study on Antiarrhythmic Activity of Experimental Example 2 Compound 2
[0075] 1. Test method
[0076] Get 125 SD rats, half male and half male, and randomly divided into 8 groups, i.e. blank solvent group (preparation method: get 4mL of 1% hydrochloric acid ethanol, add normal saline to dilute to 100mL), model control group (aconitine; dose 0.03mg / kg), positive control group (lidocaine group, dose 5mg / kg; propafenone group, dose 3.2mg / kg), compound 2 different dose groups (0.05mg / kg, 0.20mg / kg, 0.30mg / kg and 0.40mg / kg). Aconitine, the positive drug and each test drug were prepared by the preparation method of the blank solvent. The blank solvent group was only given an equal volume of the blank solvent to observe whether the solvent had an effect on the ECG of SD rats.
[0077] Before the start of the experiment, SD rats were anesthetized by intraperitoneal injection of 20% urethane (dose 1.2g / kg), fixed in the supine position, needle-shaped electrodes were inserted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com