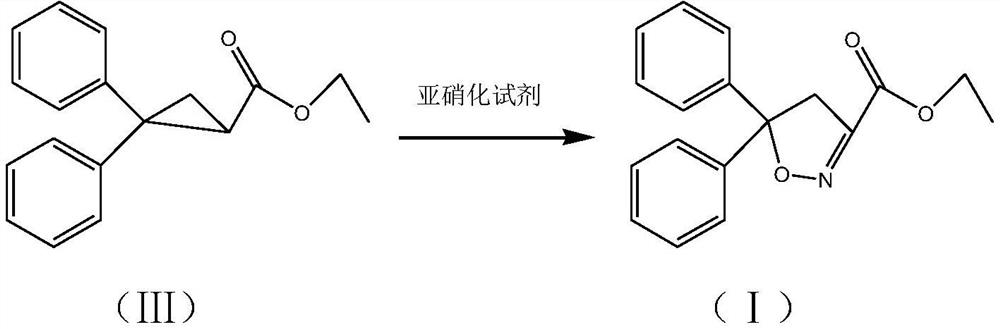
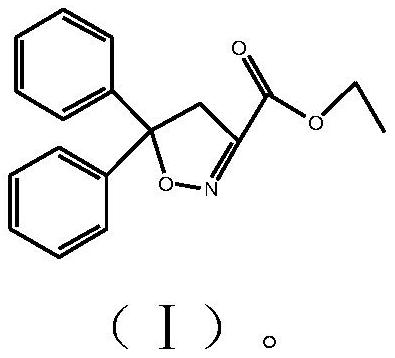
Isoxadifen-ethyl with few impurities and preparation method of ethyl isoxadifen-ethyl
A technology of ethyl bisoxazole and impurities, applied in the field of ethyl bisoxazole and its preparation, can solve problems such as being unsuitable for large-scale industrial production, low reaction safety, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
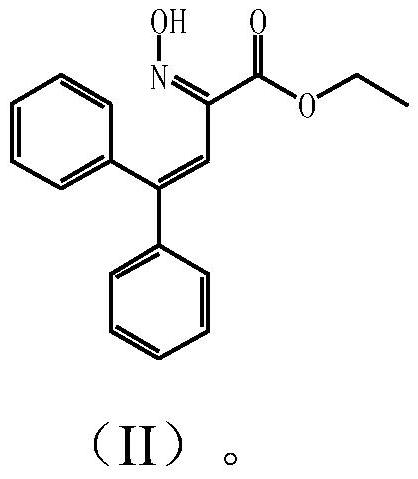
[0060] Add ethanol to the reddish-brown solid product obtained in the above comparative example, stir at 15-20°C for 3 hours, then cool down to crystallize, filter, wash the filter cake with a small amount of ethanol, and dry at 50-55°C to obtain a slightly yellow solid. The determination was carried out by high performance liquid chromatography under the same conditions as described in the comparative examples. It is measured that the content of the impurity compound represented by the above formula (II) in the solid is less than 0.5% by weight.
Embodiment 2
[0062]Prepare with reference to Examples 5 and 6 of US2016060223A1. After obtaining the reaction product containing a reddish-brown oil layer, add 3 to 10 times the weight of ethanol to it and heat up to reflux for 0.5 hours, then cool down to below 15°C to crystallize for 1 hour, filter, filter The cake was washed with a small amount of ethanol and dried at 50-55°C to obtain a white solid. The determination was carried out by high performance liquid chromatography under the same conditions as described in the comparative examples. It is measured that the content of the impurity compound represented by the above formula (II) in the solid is less than 0.2% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com