Application of vesicle in preparation of hemophilia medicine
A technology for hemophilia and vesicles, applied in the field of biomedicine, can solve the problems of easy production of autoantibodies, high treatment cost, and inability to become a treatment method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
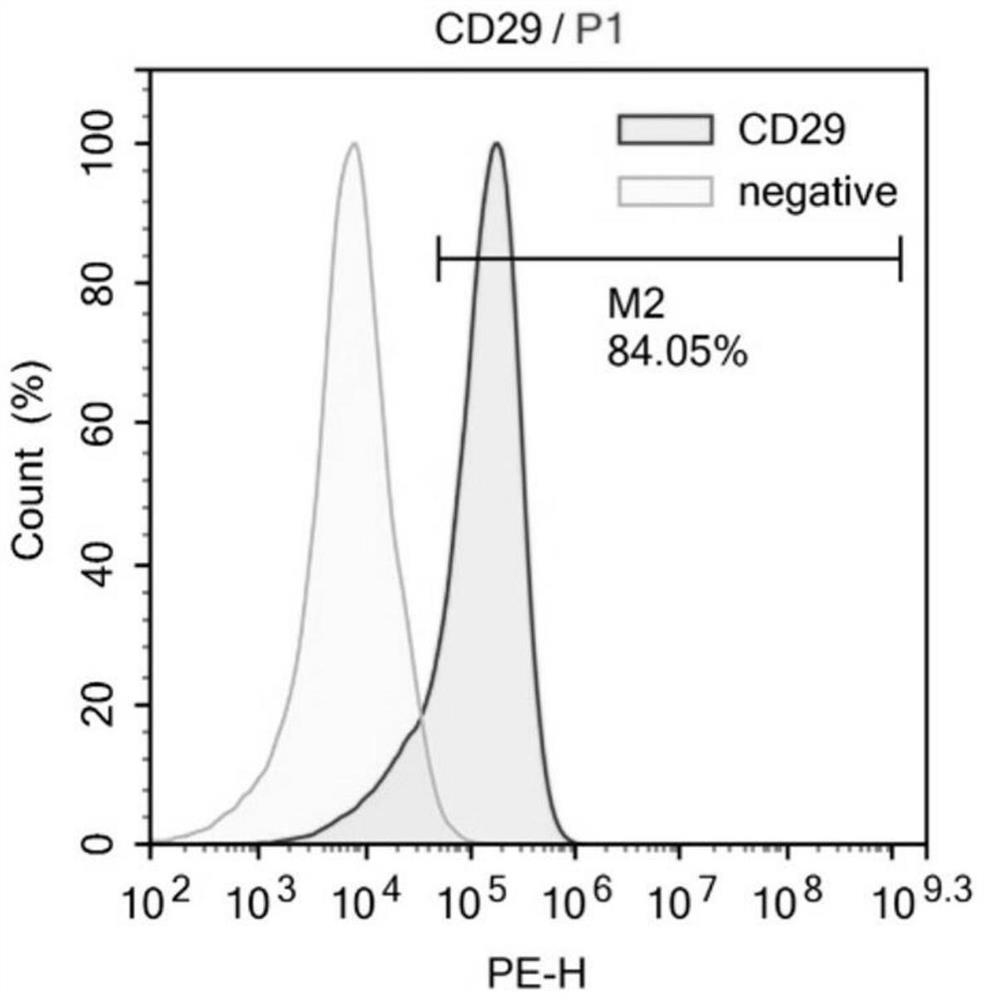
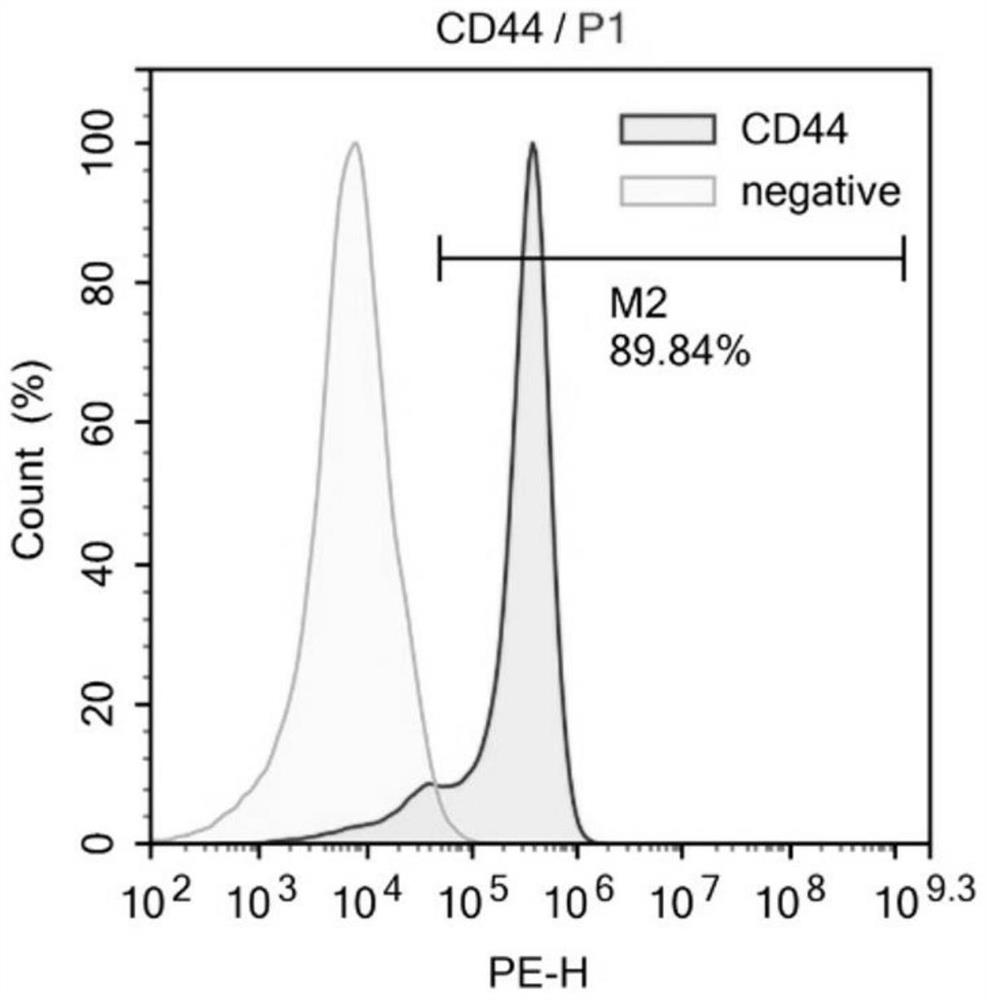
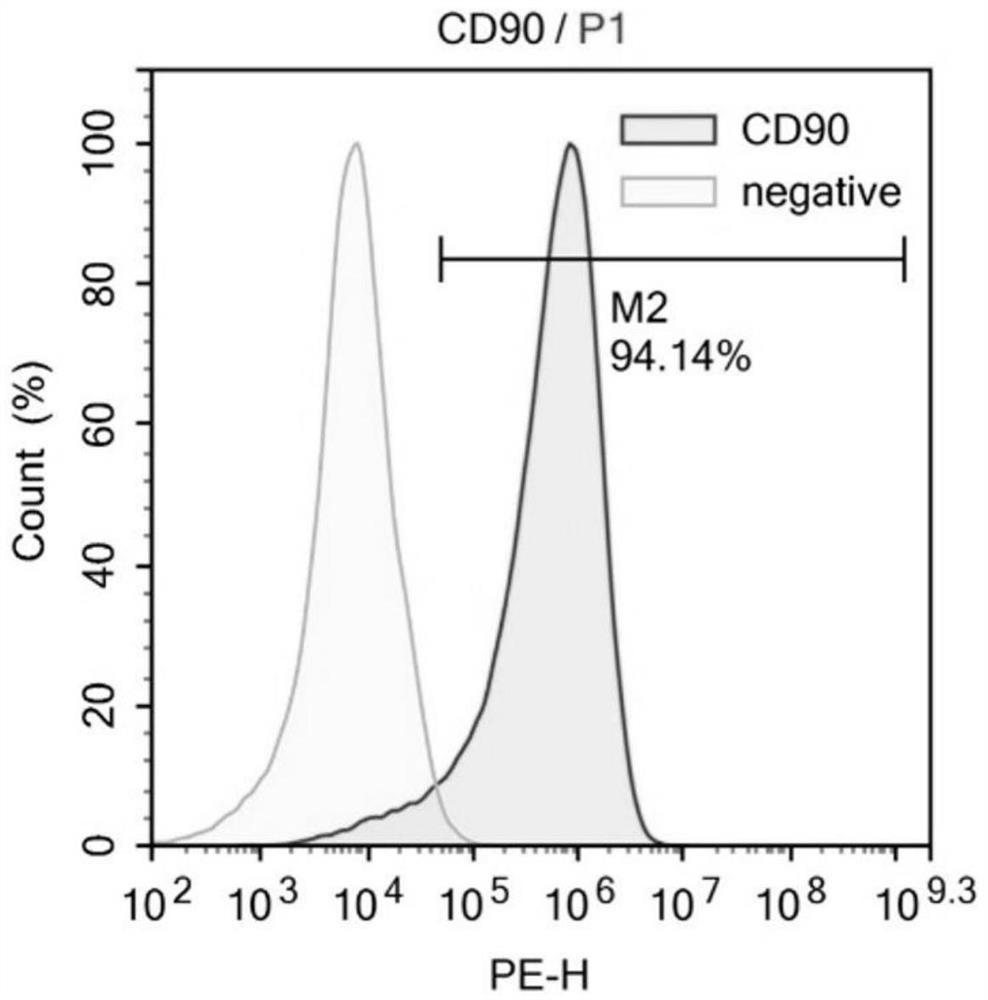
[0068] The isolation culture of embodiment 1 MSCs
[0069] Excess CO was used according to the guidelines of the Animal Ethics Committee 2 Sacrifice the mice, remove the tibia and femur under aseptic conditions, peel off the muscles and connective tissues attached to them, further separate the metaphysis, expose the bone marrow cavity, and extract the volume fraction of 10% with a 10mL sterile syringe. The bone marrow cavity was washed repeatedly with PBS of fetal bovine serum, filtered through a cell strainer with a pore size of 70 μm, and centrifuged at 500 g for 5 min. After removing the supernatant, the cell pellet at the bottom was collected, resuspended in PBS, and centrifuged again at 500 g for 5 min to collect the final cell pellet. Then the cells were sorted by flow cytometry, and BMMSCs were sorted out using CD34- and CD90+ as sorting criteria. Finally, resuspend the cells in Dex(-) medium and inoculate them in a 10cm diameter cell culture dish at 37°C, 5% CO 2 nou...
Embodiment 2
[0076] Example 2 Obtaining of inducible vesicles
[0077] The MSCs (bone marrow-derived MSCs) cultured to the second generation in Example 1 were continued to be cultured with the medium (Dex(+) medium) in Example 1 until the cells were confluent to 80%-90%, then rinsed with PBS 2 times, adding serum-free medium (α-MEM medium) containing 500nM STS to induce apoptosis, incubating at 37°C for 24h, collecting the cell supernatant for isolation and extraction of IEVs.
[0078] Separation and extraction of IEVs from the collected medium supernatant, the operation process is as follows figure 2 As shown, the specific steps include: centrifuge at 800g for 10 minutes—collect supernatant—centrifuge at 2000g for 10 minutes—collect supernatant—centrifuge at 16,000g for 30 minutes—remove supernatant, resuspend IEVs in sterile PBS—centrifuge at 16,000g for 30 minutes— Remove the supernatant and resuspend IEVs in 300-500ul sterile PBS.
Embodiment 3
[0082] The analysis of embodiment 3 IEVs
[0083] (1) Quantification of IEVs and analysis of membrane proteins
[0084] Utilize flow cytometry to carry out quantitative analysis to the IEVs that embodiment 2 obtains, measurement time point is the 1st, the 4th, the 8th, the 16th and the 24th h, the result shows 10 6 MSCs can produce 0.76×10 after induction to 1h, 4h, 8h, 16h and 24h respectively 8 pcs, 1.29×10 8 pcs, 1.95×10 8 pcs, 2.48×10 8 pcs, 3.14×10 8 IEVs, it can be seen that, after induction to 24h, a single MSC can produce 300 IEVs ( image 3 ).
[0085] In addition, flow cytometry found that the particle diameter distribution of IEVs was concentrated below 1 μm, accounting for 94.97% ( Figure 4A ).
[0086] The results of side scattered light (SSC) analysis also showed that the scattered light intensity of IEVs was concentrated in the range below 1 μm ( Figure 4B ).
[0087] Further, the scattered light intensity of IEVs was analyzed by the standardized sma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com