Pharmaceutical composition containing berberine and application of pharmaceutical composition
A composition, the technology of berberine, applied in the field of pharmaceutical composition for the treatment of hyperlipidemia, achieves the effects of reducing the dosage of a single drug, significant curative effect, and low liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0022] Embodiment 1-2: the preparation of berberine, Bempedoic Acid composite sheet (1000)
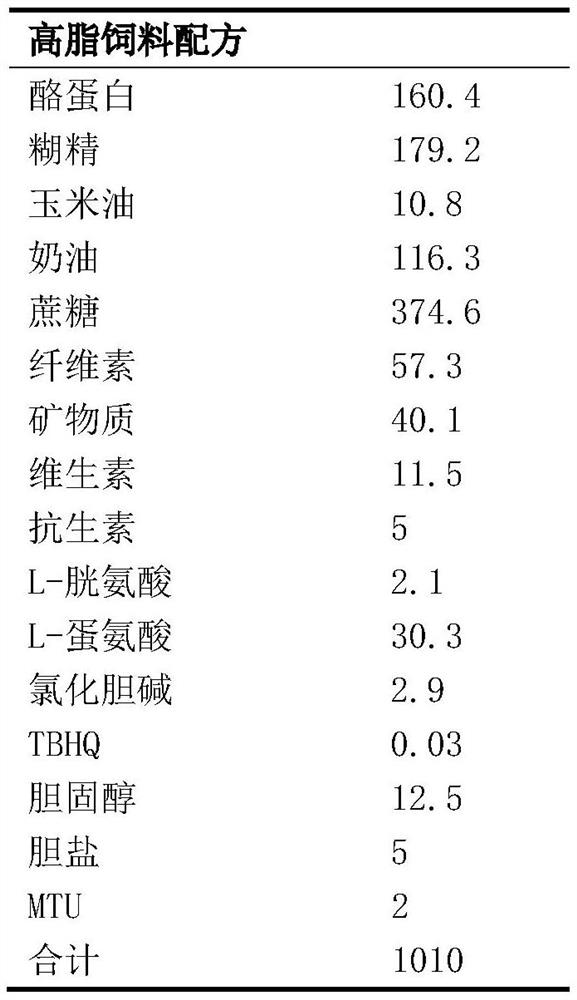
[0023] Formula composition Example 1 Example 2 berberine 50g 100g Bempedoic Acid 120g 180g pregelatinized starch 46g 50g microcrystalline cellulose 68g 72g Carboxymethyl Starch Sodium 3g 5g Sodium dodecyl sulfate 2.6g 3.6g 10% Povidone K30 Appropriate amount Appropriate amount Magnesium stearate 1.2g 1.2g
[0024] Preparation process: mix berberine and Bempedoic Acid, add sodium carboxymethyl starch and sodium lauryl sulfate, then add microcrystalline cellulose and pregelatinized starch, mix evenly, and use an appropriate amount of 10% povidone K30 ethanol The solution is made into a soft material, granulated, dried, and granulated, and the granules with a water content of about 3% are evenly mixed with an appropriate amount of magnesium stearate, and compressed into 1,000 tablets.
Embodiment 3-4
[0025] Embodiment 3-4: Preparation of berberine+Bempedoic Acid composite capsules (1000 capsules)
[0026] Formula composition Example 3 Example 4 berberine 50g 100g Bempedoic Acid 150g 150g lactose 60g 66g microcrystalline cellulose 85g 90g Sodium dodecyl sulfate 8g 8g Croscarmellose Sodium 4.5g ,5.0g 5% hypromellose solution Appropriate amount Appropriate amount Magnesium stearate 2g 2g
[0027] Preparation process: pass sodium carboxymethyl starch through a 100-mesh sieve, and pass lactose and microcrystalline cellulose through a 80-mesh sieve for later use; mix the raw material with croscarmellose sodium and sodium lauryl sulfate, and then add microcrystalline Mix cellulose and lactose, granulate with 5% hypromellose solution, dry at 50-60°C for 2 hours, mix the prepared material with magnesium stearate, and fill it with No. 1 capsule. Make 1000 capsules.
Embodiment 5--6
[0028] Embodiment 5--6: the preparation of berberine+Bempedoic Acid granule (1000 bag quantities)
[0029] Formula composition Example 5 Example 6 berberine 50g 100g Bempedoic Acid 120g 180g lactose 80g 80g pregelatinized starch 100g 100g Carboxymethyl Starch Sodium 15g, 15g aspartame 5g 5g 5% Povidone K-30 Appropriate amount Appropriate amount Magnesium stearate 0.5% 0.5%
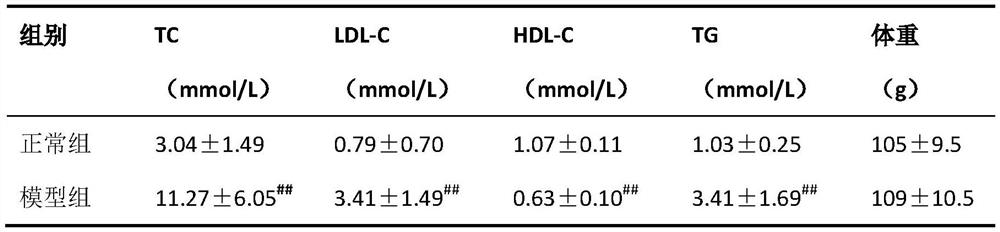
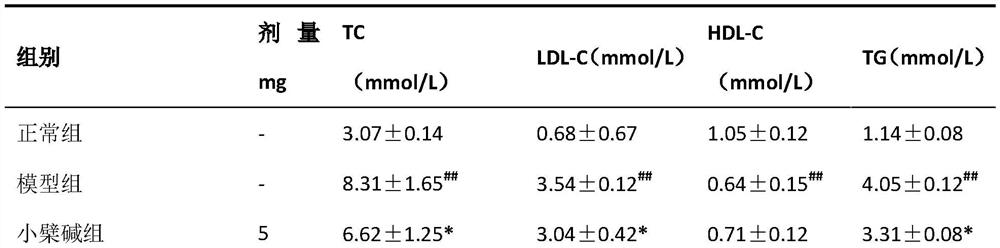
[0030] Preparation process: (1) Weigh the prescribed amount of berberine and Bempedoic Acid, pass through a 100-mesh sieve and mix them evenly for later use; (2) Pass other auxiliary materials through a 100-mesh sieve respectively for later use; (3) Weigh out the prescribed amount of lactose, Pregelatinized starch, carboxymethyl starch sodium and aspartame are mixed evenly and then mixed with the mixed raw materials; (4) Add appropriate amount of binder to make soft material, granulate with 24 mesh sieve, and dry at 40-45°C (5) 20-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com