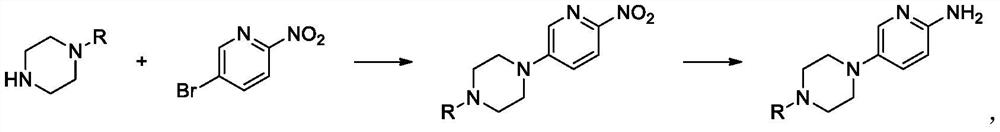
Method for preparing piperazine pyridine compound through continuous reaction
A technology of piperazine pyridine and compounds, applied in the field of pharmaceutical synthesis, can solve problems such as safety, stability and high-efficiency reaction, low heat transfer efficiency, and overflow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
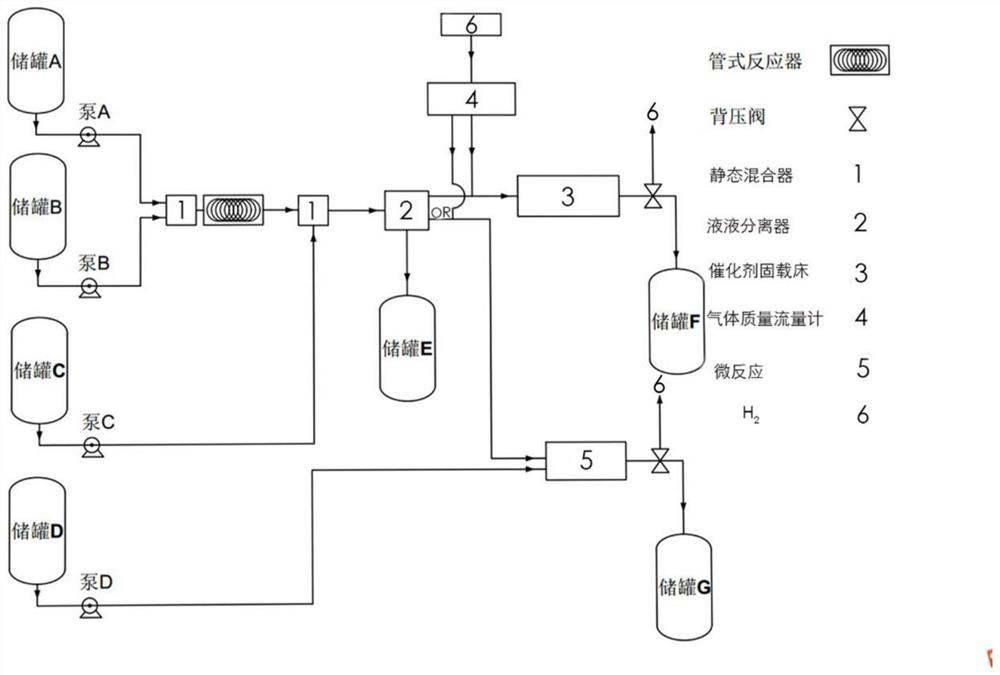
[0021] The prepared piperazine triethylamine compound solution is pumped into the static mixer and the pipeline reactor through the pump A and the pyridine solution through the pump B in proportion to control the temperature at 20°C, and the reaction time in the pipeline reactor is 240 seconds to carry out the condensation reaction. After 240 seconds, the quenched washing liquid is pumped into the static mixer for on-line mixing and quenching washing through the pump C, and then the on-line separation is carried out through the liquid-liquid separator, and the waste liquid phase enters the storage tank E. The organic phase enters the catalyst fixed-bed reactor, and at the same time, the hydrogen enters the fixed-bed reactor through the gas mass flow meter at a rate of 1.05 eq, and the hydrogen is first adsorbed on the surface by the palladium carbon of the fixed bed to form a fixed bed. The hydrogenation function back pressure valve controls the pressure to 0.5MPa, the reaction...
Embodiment 2
[0023] The prepared piperazine triethylamine compound solution is pumped into the static mixer and the pipeline reactor through the pump A and the pyridine solution through the pump B in proportion to control the temperature at 40°C, and the reaction time in the pipeline reactor is 160 seconds to carry out the condensation reaction. After 160 seconds, the quenching washing liquid is pumped into the static mixer for online mixing and quenching washing through the pump C, and then separated online by the liquid-liquid separator, and the waste liquid phase enters the storage tank E. The organic phase enters the catalyst fixed-bed reactor, and at the same time, the hydrogen gas enters the fixed-bed reactor at a rate of 1.2 eq through the gas mass flow meter, and the hydrogen is first adsorbed on the surface by the palladium carbon of the fixed bed to form a fixed bed. The hydrogenation function back pressure valve controls the pressure to 0.8MPa, the reaction temperature is control...
Embodiment 3
[0025] The prepared piperazine triethylamine compound solution is pumped through pump A and pyridine compound solution through pump B in proportion to the static mixer and pipeline reactor at the same time to control the temperature at 60°C. The reaction time in the pipeline reactor is 80 seconds for condensation reaction. The quenched washing liquid is pumped into the static mixer after 80 seconds through the pump C for online mixing and quenching washing, and then separated online by the liquid-liquid separator, and the waste liquid phase enters the storage tank E. The organic phase enters the catalyst-supported bed reactor, and at the same time, the hydrogen gas first enters the fixed-bed reactor through the gas mass flow meter at a rate of 1.4 eq, and the hydrogen is first adsorbed on the surface by the palladium carbon of the fixed-supported bed to form a fixed-supported bed. The hydrogenation function back pressure valve controls the pressure to 1.2MPa, the reaction tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com