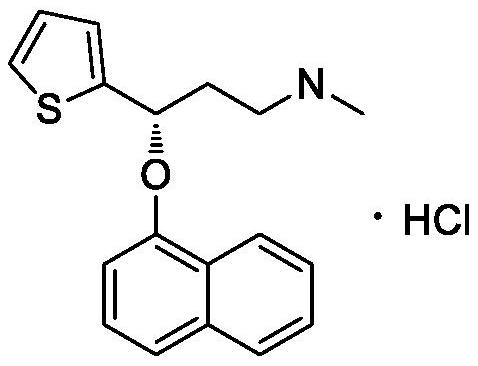
Duloxetine hydrochloride enteric-coated tablet and preparation method thereof
A technology of enteric and hydrochloric acid degree of loxetine, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as poor dissolution rate and achieve the effect of good dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
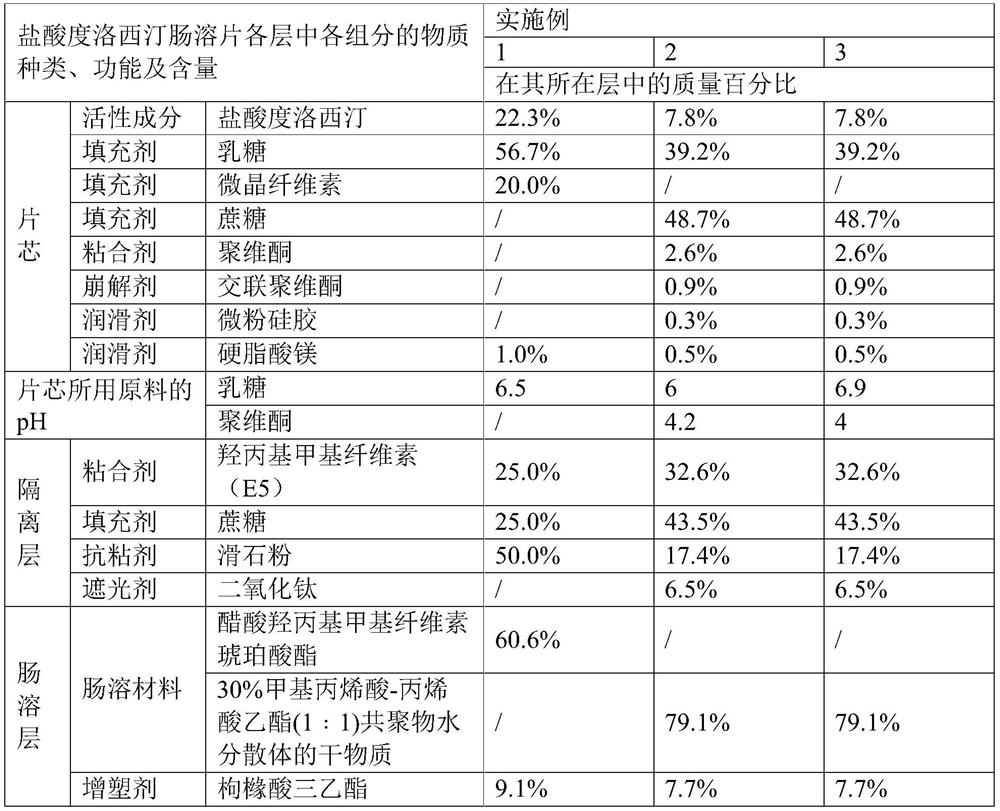
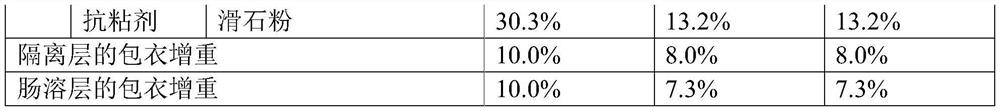
[0083] The raw materials of duloxetine hydrochloride enteric-coated tablets are prepared according to the above table, and the preparation method is as follows:
[0084] (1) Preparation of tablet cores: The active ingredients and fillers were premixed by hand, passed through a 40-mesh sieve, poured into a fast stirring granulator, and premixed for 5 minutes with the stirring paddle turned on. Then turn on the flying knife at low speed and add purified water. The mass ratio of purified water to tablet core material is 1:5. After the purified water is added, turn on the flying knife at high speed and continue granulating for about 3-8 minutes. The material is dried in an oven at 50° C., and the drying is stopped when the moisture content is less than 1.0 wt%. Install a stainless steel sieve ring with a size of 1.2mm to 2.0mm for sizing, then add a lubricant and mix for 5 minutes, press into tablets, control the hardness to 4-10kg, the friability is less than 0.8%, and the weight...
Embodiment 2-3
[0088] The raw materials of duloxetine hydrochloride enteric-coated tablets are prepared according to the above table, and the preparation method is as follows:
[0089] (1) Preparation of tablet core: pretreatment: powder sucrose and pass through a 40-mesh sieve, and the remaining raw materials are passed through a 40-mesh sieve in turn. Wet granulation: ①Preparation of binder: Dissolve the binder in 70% (w / w) ethanol aqueous solution, and the mass ratio of the ethanol aqueous solution to the tablet core raw material is 1:18. ②Put the active ingredients and fillers into the tank mixer, mix for 10 minutes, pour in the binder evenly, and continue stirring for 3 to 5 minutes after pouring to make a soft material, and turn off the stirring. ③ Install a 20-mesh nylon net on the swing granulator, add the soft materials into the hopper in stages, and carry out the granulation operation. Collect the prepared wet granules into the stainless steel basin, and then evenly and appropriate...
Embodiment 4-7
[0093] The raw materials of duloxetine hydrochloride enteric-coated tablets were prepared according to the following table, and the preparation method was the same as in Example 1.
[0094]
[0095]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com