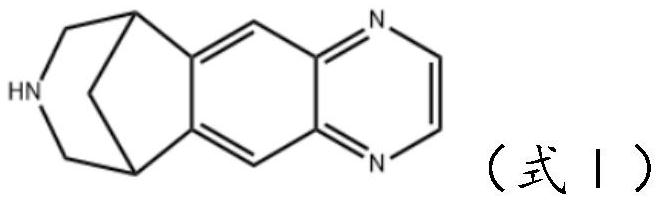
Varenicline transdermal solution as well as preparation method and application thereof
A varenicline and solution technology, applied in the field of medicine, can solve the problems of reducing drug compliance of smoking cessation patients, inability to concentrate, and affecting the effect of smoking cessation, so as to shorten the production cycle, avoid gastrointestinal side effects, and improve patient compliance Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
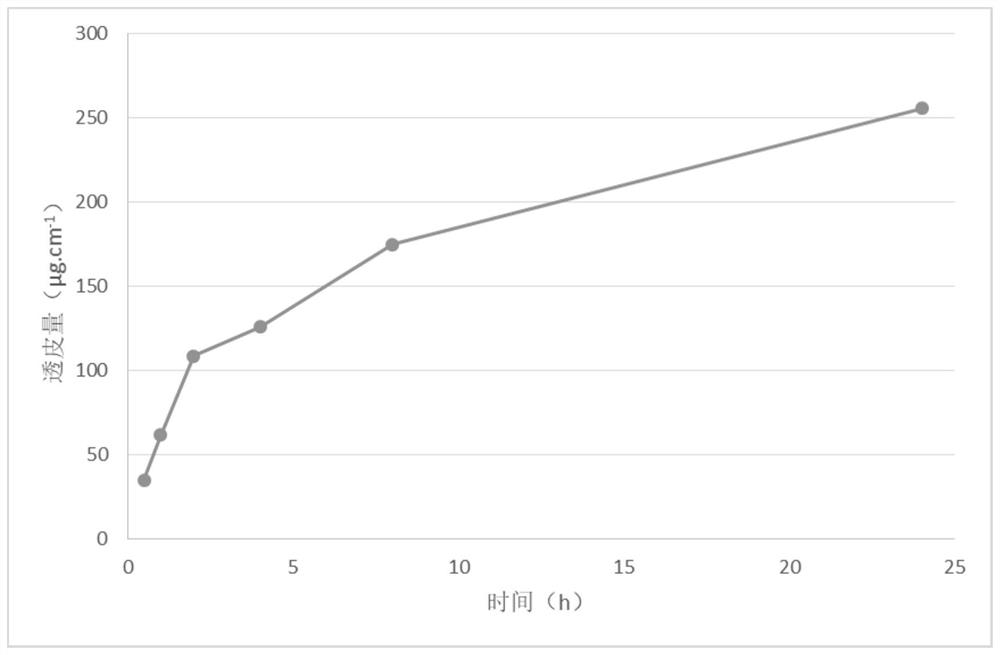
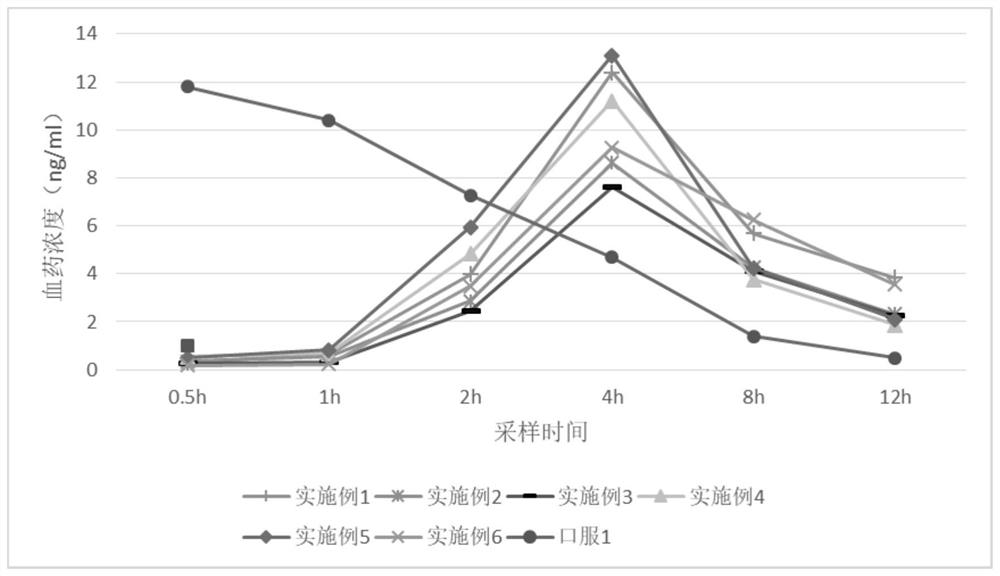
Examples
Embodiment 1
[0076] The preparation of embodiment 1 varenicline transdermal solution
[0077] Composition of Varenicline transdermal solution:
[0078]
[0079] The preparation method of varenicline transdermal solution, comprises the steps:
[0080] 1) Weigh the required amount of varenicline tartrate, and disperse it in glycerin to prepare component one;
[0081] 2) Weigh the required amount of propylene glycol, potassium sorbate, and disodium hydrogen phosphate, and dissolve it in the required amount of purified water to obtain component two;
[0082] 3) Mix component 1 and component 2 uniformly, add appropriate amount of sodium hydroxide, adjust pH to 5, and obtain the product.
Embodiment 2-4
[0083] The preparation of embodiment 2-4 varenicline solution
[0084] Composition of Varenicline transdermal solution:
[0085]
[0086]
[0087] The preparation method of varenicline transdermal solution, comprises the steps:
[0088] 1) Weigh the required amount of varenicline tartrate, and disperse it in glycerin to prepare component one;
[0089] 2) Weigh the required amount of propylene glycol, potassium sorbate, and sodium citrate, and dissolve them in the required amount of purified water to obtain component two;
[0090] 3) Mix component 1 and component 2 uniformly, add appropriate amount of citric acid to adjust pH 3.57, pH 7.71, and pH 8.96, respectively.
Embodiment 5
[0091] The preparation of embodiment 5 varenicline solution
[0092] Composition of Varenicline transdermal solution:
[0093]
[0094] The preparation method of varenicline transdermal solution, comprises the steps:
[0095] 1) Weigh the required amount of varenicline tartrate, and disperse it in glycerin to prepare component one;
[0096] 2) Weigh the required amount of propylene glycol, potassium sorbate, and sodium acetate, and dissolve it in the required amount of purified water to obtain component two;
[0097] 3) Mix component 1 and component 2 uniformly, add appropriate amount of glacial acetic acid, adjust pH to 5, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com