Anthraquinone/coumarin dimer novel skeleton compound, and preparation method and application thereof
The technology of a skeleton compound, coumarin, is applied in the fields of organic chemistry, drug combination, metabolic diseases, etc., and can solve problems such as unseen structures, preparation methods and application reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 ruby euphorquinone A
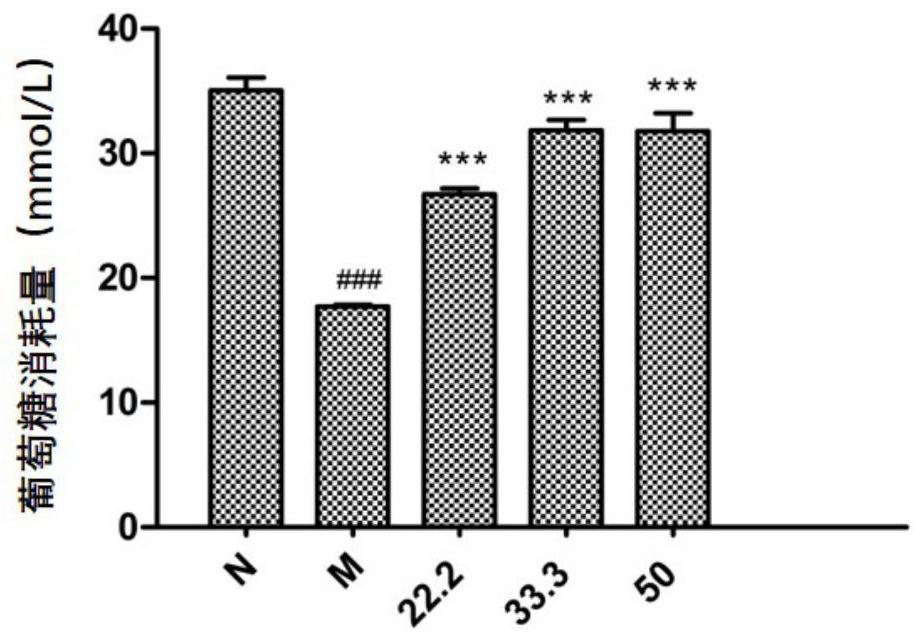
[0026] Red Euphorbia 20Kg, pulverized and ultrasonically extracted with 95% ethanol at room temperature, the extracts were combined and evaporated to dryness under reduced pressure to obtain 3.9Kg of extract. After the extract was dissolved in water, it was extracted three times with ethyl acetate, and the solvent was evaporated to obtain 400 g of ethyl acetate extract. The ethyl acetate fraction was separated by silica gel column chromatography, and eluted with petroleum ether / acetone at volume ratios of 10:0, 10:1, 10:2, 2:1, 1:1, and 0:1 in sequence. Petroleum ether / acetone 2:1 eluted fraction (30g) was separated by silica gel column chromatography again, and washed with chloroform / methanol with a volume ratio of 100:1, 10:1, 2:1, 1:1, and 0:1 in sequence take off. Chloroform / methanol 1:1 eluted fraction (4.7g) was separated by MCI column chromatography, and eluted with 30%, 50%, 70%, 90%, and 100% methanol / w...
Embodiment 2
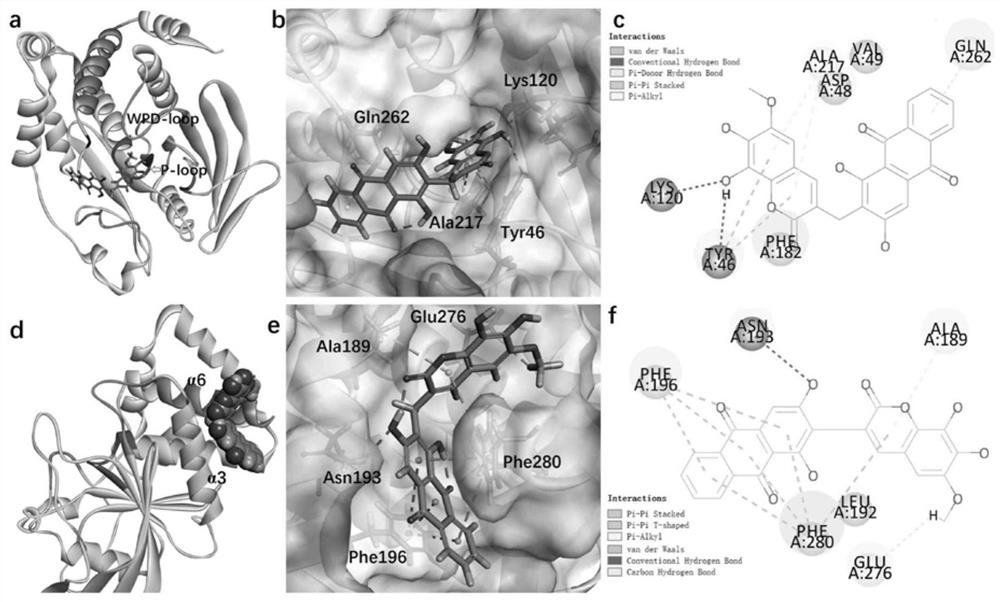
[0028] Example 2 PTP1B and TCPTP inhibitory activity evaluation
[0029] Compounds were prepared in gradient concentration with DMSO (final concentrations were 50, 25, 12.5, 6.2, 3.1, 1.6 μM, respectively). Add the pre-prepared enzyme solution (buffer containing 25mM Hepes pH 7.5, 150mM NaCl, 5mM DTT, 2mM EDTA, containing 0.1μg PTP1B or 0.15μg TCPTP) into the 96-well plate, 90μL per well, add 1μL of the compound solution, Using DMSO as a blank control, three parallel wells were set up for each concentration, and the 96-well plate was placed in a constant temperature shaker and shaken at 37°C for 15 minutes. After 15 minutes, 10 μL of p-NPP solution with a final concentration of 4 mM was added, and shaken in a constant temperature shaker at 37° C. for 30 minutes. Take it out after 30 minutes, add 20 μL 3M NaOH solution in turn to terminate the reaction, and finally measure the OD value at 405 nm in a microplate reader, and calculate the inhibitory rate and IC of the compound o...
Embodiment 3
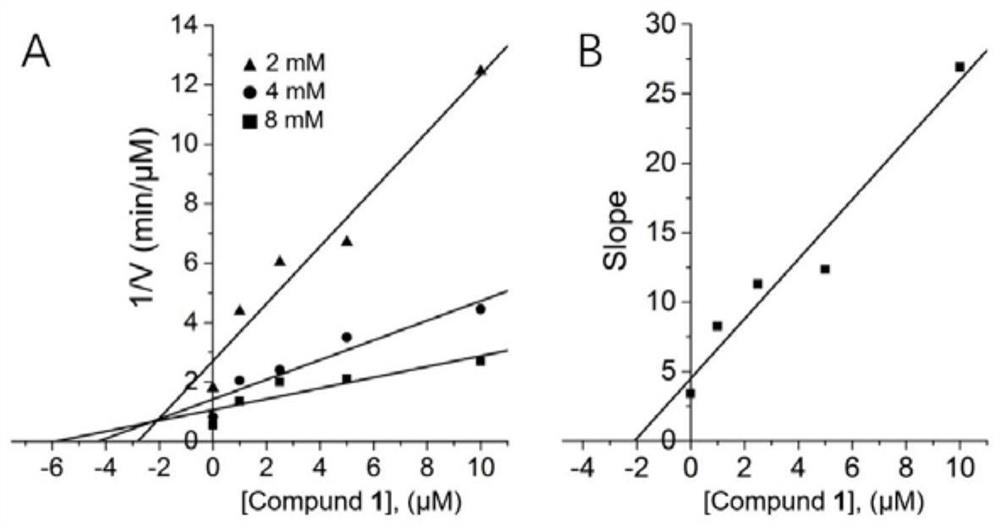
[0035] Example 3 PTP1B enzyme inhibition kinetics experiment
[0036] Add the pre-prepared PTP1B solution into a 96-well plate, 90 μL per well, add 1 μL of the compound solution to be tested, and the final concentrations are 0, 1, 2.5, 5, and 10 μM, respectively, and set up three replicate wells for each concentration. As a blank control, the 96-well plate was placed in a constant temperature shaker and shaken at 37°C for 15 minutes. Then add p-NPP solution (final concentrations are 2, 4, and 8 mM respectively), and immediately use a microplate reader to measure the change of OD value at 405 nm within 15 min, and calculate the initial rate of enzymolysis. The concentration of the compound and the initial rate of enzymolysis were plotted by Dixon plot, and the slope of the straight line at each concentration obtained by Lineweaver-Burk plot was plotted by the concentration of the compound and the slope to obtain the value of the inhibition constant Ki. The type of inhibition o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com