Pantolactone hydrolase mutant strain and application thereof
A pantolactone and hydrolase technology, applied in the field of pantolactone hydrolase mutant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
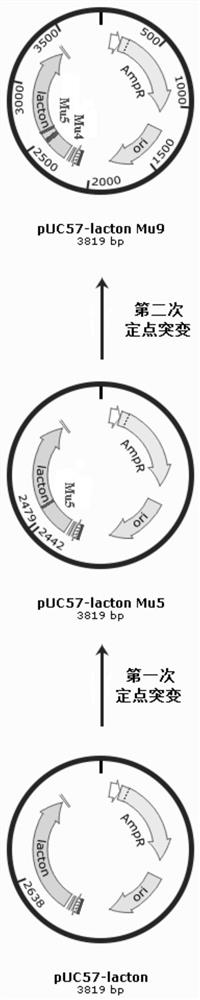
[0118] Construction of the genetically engineered strain AR / pHT01-P43-lacton producing D-pantolactone hydrolase 1. Synthetic cloning vector pUC57-lacton:
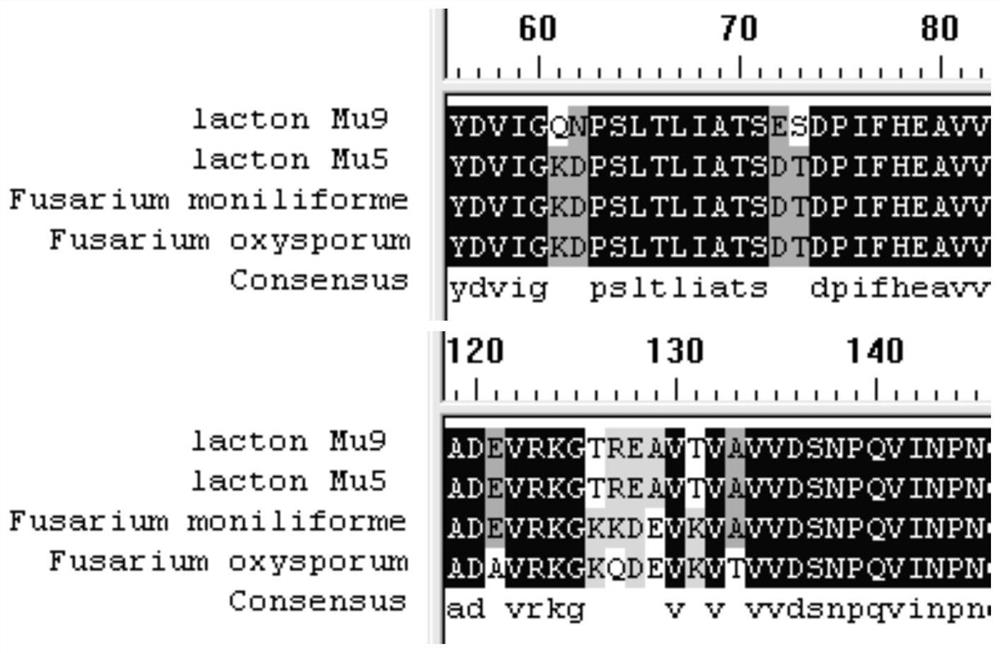
[0119] Referring to the D-pantolactone hydrolase gene of Fusarium oxysporum (Fusarium oxysporum, GenBank: AB010465) and Fusarium moniliforme (Fusarium moniliforme, GenBank: AY728018), the nucleotide sequences are respectively as SEQ ID NO: 4 and 5. Submit the nucleotide sequence to Suzhou Jinweizhi Biotechnology Co., Ltd. for codon optimization and synthesis of the cloning vector pUC57-lacton. The optimized sequence is shown in sequence SEQ ID NO:6, and the corresponding amino acid sequence is shown in sequence SEQ ID NO:1.
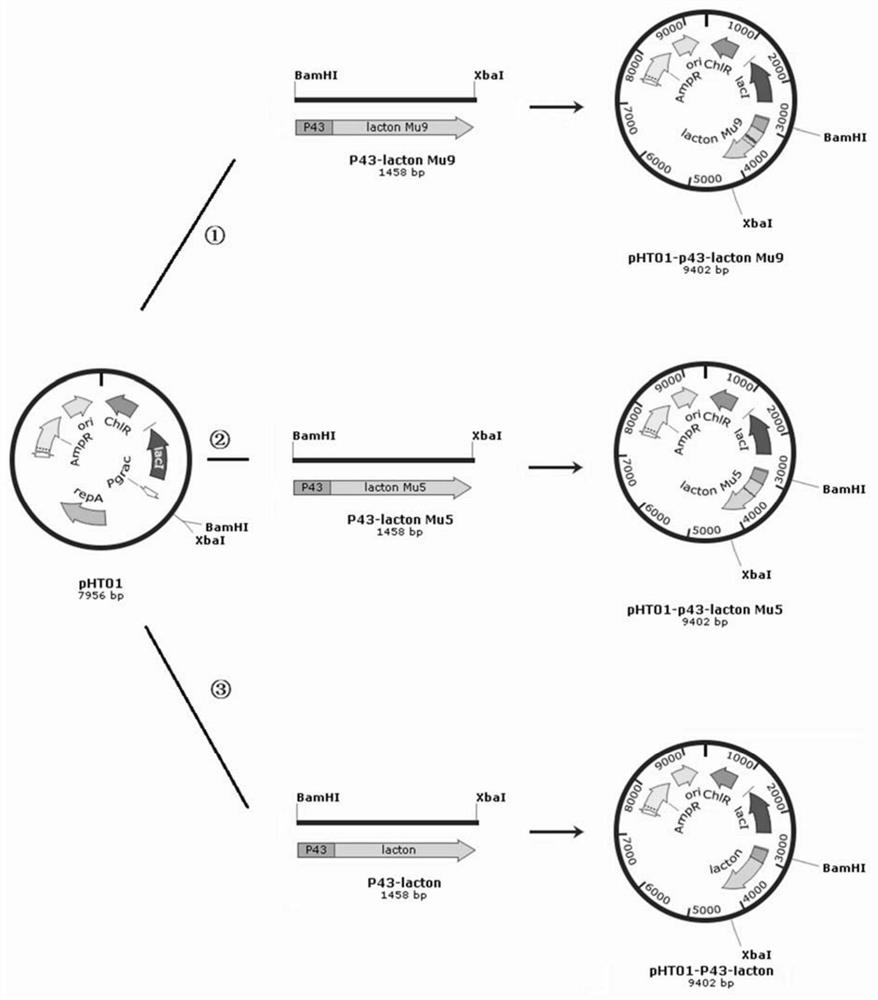
[0120] 2. Construction of expression vector pHT01-P43-lacton
[0121] Amplify the P43 promoter and lacton sequence, obtain P43-lacton by overlapping, digest the vector pHT01 and P43-lacton with BamHI and XbaI respectively, connect them, transform them into E.coli DH10B, and place them on a plate with...
Embodiment 2
[0127] Construction of the genetically engineered strain AR / pHT01-P43-lacton Mu5 producing D-pantolactone hydrolase 1. Construction of the cloning vector pUC57-lacton Mu5:
[0128] Using the plasmid pUC57-lacton as a template, primers lac-mutF1 / lac-mutR1 (see Table 9 for the sequence), PCR amplification was performed for site-directed mutagenesis, and the recovered amplified product was digested with DpnI enzyme at 37°C for 1 hour, and transferred to Ecoli XL1blue competent Cells were cultured overnight at 37° C. on a plate containing 100 mg / L ampicillin antibiotic, and positive clones were obtained by screening. The specific nucleotide sequence is shown in SEQ ID NO: 7, and the corresponding amino acid sequence is shown in SEQ ID NO: 2.
[0129] 2. Construction of expression vector pHT01-P43-lacton Mu5
[0130] Amplify the P43 promoter and lacton sequence, obtain P43-lacton Mu5 by overlapping, digest the vector pHT01 and P43-lacton Mu5 with BamHI and XbaI respectively, connec...
Embodiment 3
[0136] Construction of Genetically Engineered Bacteria AR / pHT01-P43-lacton Mu9 Producing D-Panttolactone Hydrolase
[0137] 1. Construct the cloning vector pUC57-lacton Mu9:
[0138] Using the plasmid pUC57-lacton Mu5 as a template, primers lac-mutF2 / lac-mutR2 (see Table 9 for the sequence), PCR amplification was performed for site-directed mutagenesis, and the recovered amplified product was digested with DpnI enzyme at 37°C for 1 hour, and transferred to Ecoli XL1 blue In the competent cells, cultivate overnight at 37°C on a plate with ampicillin antibiotic 100 mg / L, and screen and identify positive clones. The specific nucleotide sequence is shown in the sequence SEQ ID NO: 8, and the corresponding amino acid sequence is shown in SEQ ID NO: 3 shown.
[0139] 2. Construction of expression vector pHT01-P43-lacton Mu9
[0140] Amplify the P43 promoter and lacton Mu9 sequence, obtain P43-lacton Mu9 by overlapping, digest the vector pHT01 and P43-lacton Mu9 with BamHI and XbaI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com