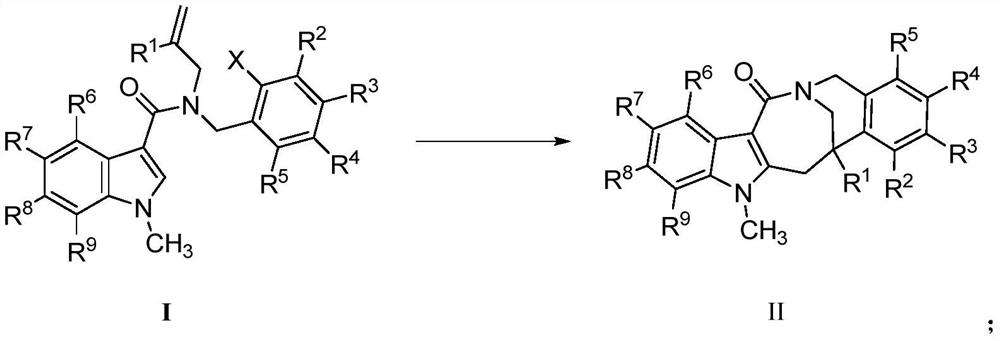
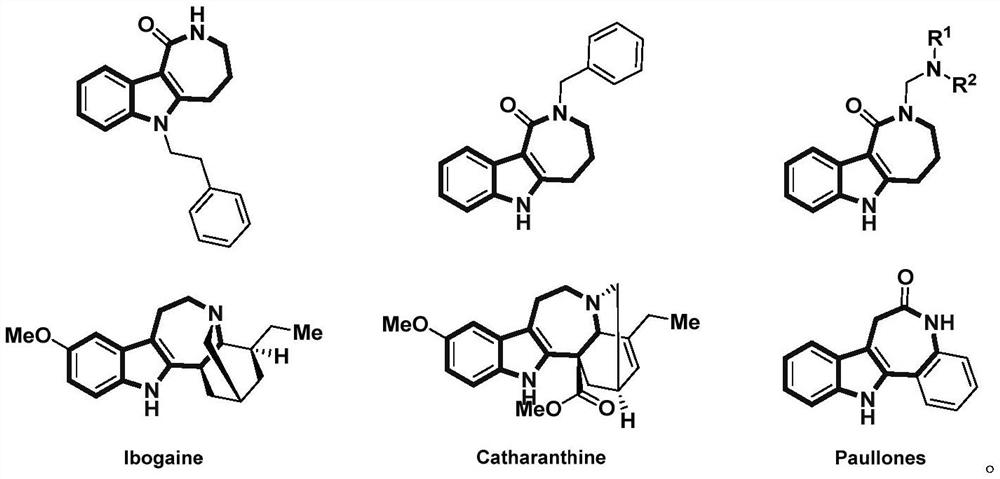
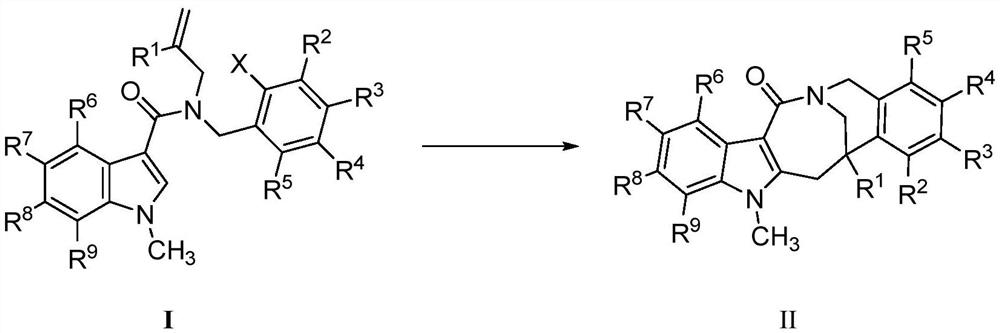
Preparation method of fused ring compound containing indole skeleton
A compound and skeleton technology, applied in the field of preparation of fused ring compounds, can solve problems such as limited construction methods, cumbersome operations, and narrow substrate applicability, and achieve the effects of wide substrate applicability, simple system, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of 5-methyl-7-phenyl-5,6,7,12-tetrahydro-14H-7,13-methylbenzo[7,8]azo[4,3-b] Indol-14-one (2a)
[0044]
[0045] Put a rotor into a 10mL dry Schlenk tube, weigh 0.2mmol of raw material 1a (1.0eq), 0.02mmol of palladium iodide (10mol%), 0.6mmol of cesium carbonate (3eq) and 0.04mmol of 4-(dimethylamino ) triphenylphosphine (20mol%) was placed in the bottle; under argon atmosphere, add ultra-dry acetonitrile / toluene (1:1, 2mL) and 1.4mmol H 2 O (7.0 equivalents), the mixture was pre-stirred at room temperature for 30min, and the reaction mixture was placed in a 100°C oil bath and stirred for 12h; after the reaction, the mixture was cooled to room temperature, diluted with ethyl acetate, filtered with diatomaceous earth, and the filtrate was vacuum Concentration gave a yellow oily liquid; the obtained crude product was separated by flash column chromatography (silica gel soaked in triethylamine, petroleum ether / ethyl acetate=4:1, v / v) to obtain t...
Embodiment 2
[0047] Example 2: Preparation of 5-methyl-7-(naphthalene-2-yl)-5,6,7,12-tetrahydro-14H-7,13-methylbenzo[7,8]azo[4 ,3-b]indol-14-one (2b)
[0048]
[0049] 1b was used as the starting substrate for the reaction to prepare the target product 2b, the reaction temperature was 100°C, and the reaction was carried out for 12 hours. The preparation method was basically the same as that in Example 1.
[0050] Product yield 78%; white solid; 1 H NMR (400MHz, CDCl 3 )δ8.09–8.00(m,1H),7.84–7.72(m,4H),7.54–7.42(m,2H),7.36–7.30(m,1H),7.28–7.14(m,7H),5.54( dd,J=16.1,2.0Hz,1H),4.51(d,J=16.1Hz,1H),4.28(dd,J=14.3,2.1Hz,1H),4.18(d,J=17.2Hz,1H), 3.89(s,3H),3.56(d,J=14.4Hz,1H),3.51(d,J=17.2Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ169.05,143.88,143.39,140.31,137.23,135.65,133.10,132.18,129.77,128.64,128.04,127.62,127.50,127.23,127.16,127.00,126.66,126.39,125.67,125.34,122.57,121.74,121.05,109.60,109.41 ,57.82,49.54,49.13,38.82,30.21.HRMS(ESI)m / z calculated for C 30 h 25 N 2 O for[M+H] + :429...
Embodiment 3
[0051] Example 3: Preparation of 5-methyl-7-(4-methoxyphenyl)-5,6,7,12-tetrahydro-14H-7,13-methylbenzo[7,8]azo [4,3-b]indol-14-one (2c)
[0052]
[0053] 1c was used as the starting substrate of the reaction to prepare the target product 2c, the reaction temperature was 100°C, and the reaction was carried out for 12 hours. The preparation method was basically the same as that in Example 1.
[0054] Product yield 76%; white solid; 1 H NMR (400MHz, CDCl 3)δ8.06–7.99(m,1H),7.34–7.29(m,1H),7.27–7.21(m,4H),7.20–7.12(m,4H),6.90–6.80(m,2H),5.50( dd,J=16.1,2.0Hz,1H),4.47(d,J=16.1Hz,1H),4.21(dd,J=14.2,2.1Hz,1H),4.03(d,J=17.3Hz,1H), 3.84(s,3H),3.79(s,3H),3.46(d,J=17.2Hz,1H),3.44(d,J=14.0Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ168.96,158.41,143.78,140.42,138.84,137.23,135.63,129.69,128.19,127.39,127.16,127.01,122.54,121.72,121.05,114.04,109.59,109.37,58.13,55.44,49.09,48.91,39.17,30.15.HRMS (ESI)m / z calculated for C 27 h 25 N 2 o 2 for[M+H] + :409.1916,found:409.1913.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com