Small molecular protein for efficiently mediating recombinant polypeptide to form inclusion body
A technology for small molecular proteins and recombinant polypeptides, applied in the field of bioengineering, can solve problems such as cumbersome operations, and achieve the effect of simplifying the separation and purification process, with significant effects, large market value and application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The construction of embodiment 1 pET28b-Am-Metch expression vector
[0046] (1) In vitro synthesis of genes encoding small molecular proteins (Am) that efficiently mediate the formation of inclusion bodies by recombinant polypeptides
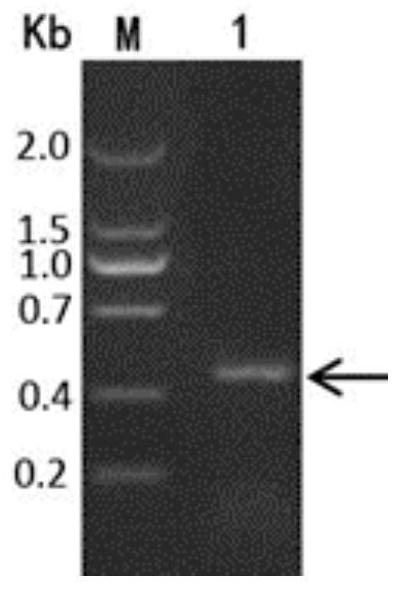
[0047] According to the hydrophobic and hydrophilic properties of different amino acids and their tendency to form β sheets, the C-terminal sequence (100-161 amino acids) of Escherichia coli palmitoylphospholipid transferase (PagP) was optimized and transformed (PagP-100), and the design was easy The fusion tag (Am) of the inclusion body was formed, and the nucleotide sequence of the gene encoding Am was designed according to the codon bias of E. coli. Wherein, the nucleotide sequence of the gene encoding Am was synthesized by Beijing Ruibo Biotechnology Co., Ltd. Guangzhou Branch.
[0048] Nucleotide sequence of PagP-100:
[0049] AATTTTCATTTAGGTCTGGGATTCACCGCTGGCGTAACGGCACGCGATAACTGGAATTACATCCCTCTCCCGGTTCTACTGCCATTGGCCTCCGTGGGTTATGGCC...
Embodiment 2
[0075] Example 2 Induced expression of recombinant polypeptides in the form of inclusion bodies in Escherichia coli
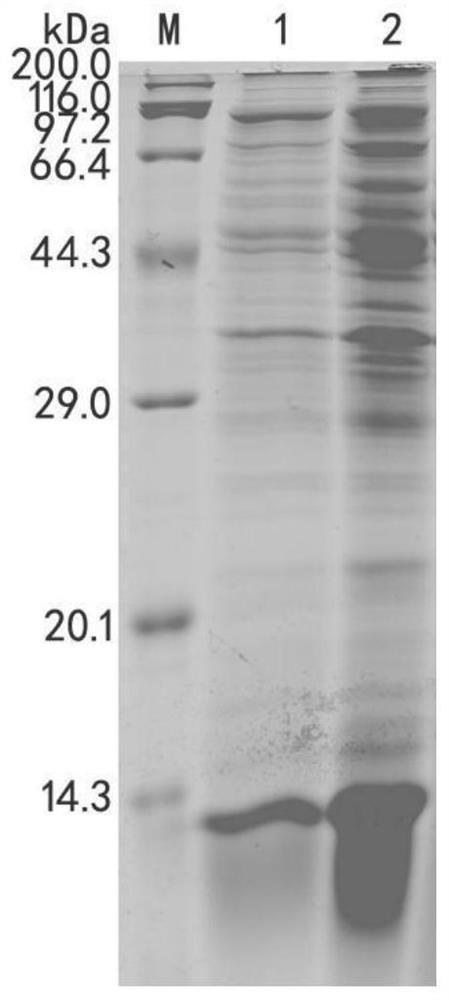
[0076] The pET28b-Am-Metch expression vector constructed above and the control pET28b-PagP-100-Metch expression vector (PagP-100 replacing Am) were transformed into Escherichia coli BL21 (DE3) competent cells, and a single clone was picked and inoculated into liquid LB Cultivate overnight in the culture medium, expand the culture to 0D at a volume ratio of 1:100 600 0.5-0.7, add isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 0.6mM, and induce protein expression at 37°C for 24h. The bacterial cells were collected by centrifugation under the conditions of 5000 g, 10 min, and 4° C. to obtain the bacterial cells expressing the recombinant polypeptide.
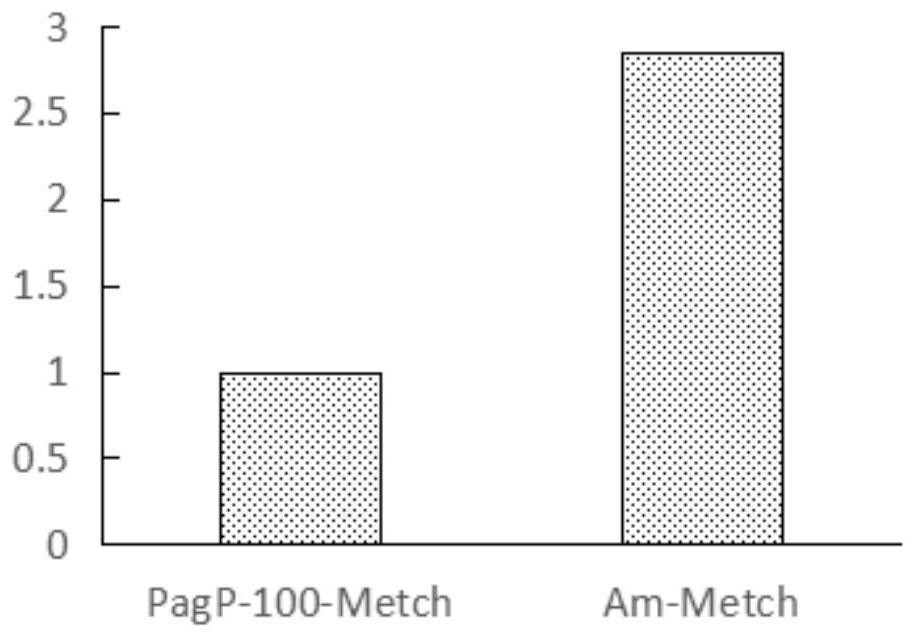
[0077] Detect the expression of the recombinant polypeptide by 18% SDS-PAGE, the results are as follows figure 2 As shown, the expression level of Am-Metch was significantly better than th...
Embodiment 3
[0080] Example 3 Dissolution of inclusion bodies and separation and purification of target polypeptide Metch
[0081] (1) Take 1×Lysis Buffer (50mmol NaH 2 PO 4 , 300mmol NaCl, 20mmol imidazole, pH8.0) Suspend the bacterium expressing the recombinant polypeptide collected in Example 2, sonicate it, and centrifuge at 5000g, 30min, 4°C to obtain the inclusion body expressing the recombinant polypeptide ;
[0082] (2) Mix the inclusion body with 100 or 200 mmol acetic acid solution at a ratio of 10 mg: 1 ml to achieve dissolution of the inclusion body. SDS-PAGE was used to detect the dissolution of inclusion bodies in the dissolved samples, such as Figure 5 As shown, the Am-Metch inclusion body dissolution effect is good after the 200mmol acetic acid solution is dissolved.
[0083] (3) Add Tween-20 at 2% volume percentage to the dissolved sample, shake at 37°C for 2h to further promote dissolution; then use NaOH to adjust the pH to 4.5, add TEV enzyme at 10% volume percentag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com