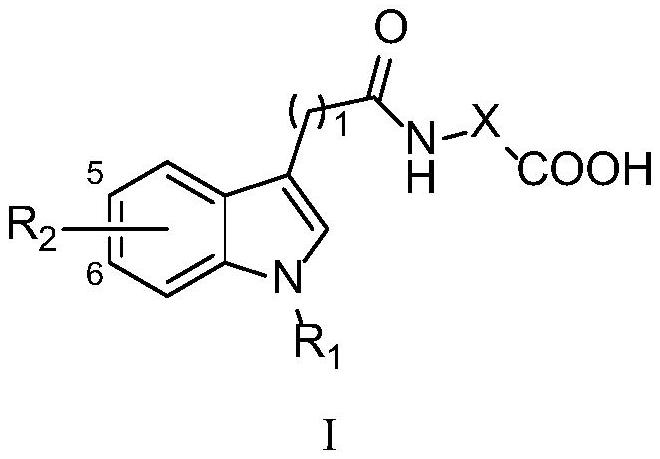
Indole derivative as well as preparation method and application thereof
A technology of indole derivatives and derivatives, applied in the field of medicine, can solve problems such as cardiovascular toxicity, liver and kidney damage, and achieve the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
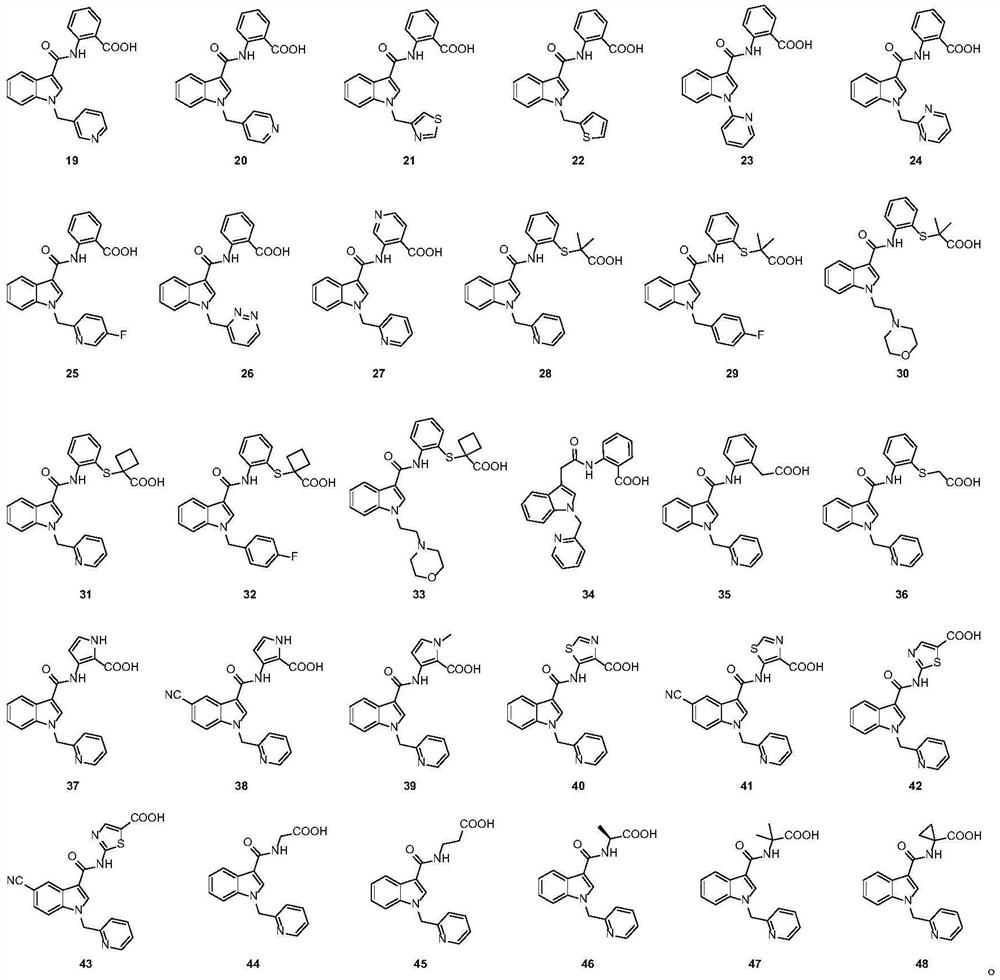
Examples
Embodiment 1
[0064] The preparation process of 2-(1H-indole-3-carboxamido)benzoic acid (compound 1) comprises:
[0065] Add 3-indolecarboxylic acid (50.00 g, 0.31 mol), 300 mL of dichloromethane, 70 mL of thionyl chloride, and 3 mL of DMF to the reaction flask in sequence, raise the temperature to reflux, and stir for 1 h. The reaction solution was evaporated to dryness under reduced pressure, and 100 mL of dichloromethane was added to evaporate to dryness again to remove residual thionyl chloride. Add 300mL of dichloromethane to make acid chloride suspension for later use. Take another 2000mL three-necked flask, add methyl anthranilate (42.63g, 0.28mol), triethylamine (32g, 0.31mol), and 500mL of dichloromethane in an ice-water bath and stir. The acid chloride suspension was added dropwise to the reaction solution, stirred for 30 min after dropping, and then turned to room temperature to react overnight. TLC monitoring, the reaction was completed, the solvent was evaporated under reduce...
Embodiment 2
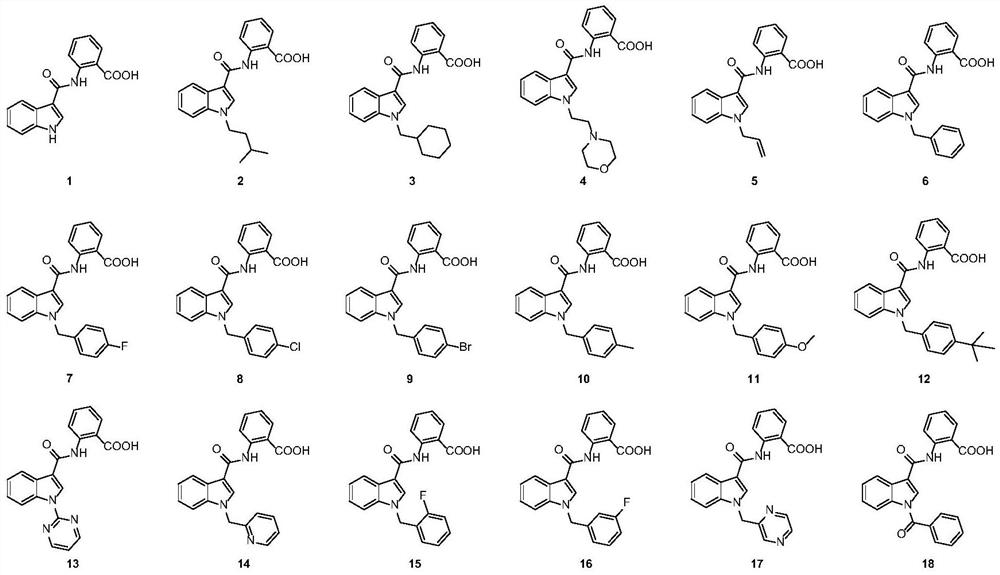
[0071] The preparation process of 2-(1-isoamyl-1H-indole-3-carboxamido)benzoic acid (compound 2) comprises:
[0072] Using 3-indolecarboxylic acid as the starting material, the intermediate compound 1C was prepared in the manner of Example 1;
[0073] Add intermediate compound 1C (2g, 6.8mmol), anhydrous potassium carbonate (1.23g, 13.6mmol), potassium iodide (0.11g, 0.68mmol) and DMF25mL to a 100ml single-necked bottle, stir at room temperature for 0.5h, and add bromine dropwise thereto Substituting isopentane (0.94g, 8.2mmol), the temperature was raised to 60°C for reaction. After the reaction was complete as monitored by TLC, the reaction solution was cooled to room temperature, the filtrate was poured into 50mL water, and ethyl acetate was added for extraction (20mL×2). The organic phases were combined, and the organic phase was washed successively with saturated sodium bicarbonate (10 mL×3) and saturated sodium chloride (10 mL×2). Add anhydrous sodium sulfate to the orga...
Embodiment 3
[0079] 2-[1-(pyridin-2-yl)-1H-indole-3-carboxamido]benzoic acid (compound 23):
[0080] Using 3-indolecarboxylic acid as the starting material, the intermediate compound 1C was prepared in the manner of Example 1;
[0081]Add intermediate compound 1C (2g, 6.8mmol), anhydrous potassium carbonate (1.97g, 14.3mmol), 2-bromopyridine (1.40g, 8.8mmol), trans-1,2-ring Hexamethylenediamine (0.15g, 1.4mmol), cuprous iodide (0.06g, 0.3mmol) and 2mL of 1,4-dioxane were heated to 110°C for 10h under nitrogen protection. After the completion of the reaction was monitored by TLC, the reaction solution was cooled to room temperature, saturated sodium chloride was added, and extracted with ethyl acetate (20 mL×2). The organic phases were combined, and the organic phase was washed successively with saturated sodium bicarbonate (10 mL×3) and saturated sodium chloride (10 mL×2). The organic phase was dried overnight by adding anhydrous sodium sulfate, filtered with suction, and evaporated to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com