A kind of z-selective ruthenium carbene olefin metathesis catalyst and its preparation method and application
A metathesis catalyst and selective technology, applied in catalytic reactions, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low catalytic activity, catalyst selectivity and activity need to be further improved, and few catalyst types, etc. The effect of improving catalytic activity, excellent thermal stability, and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
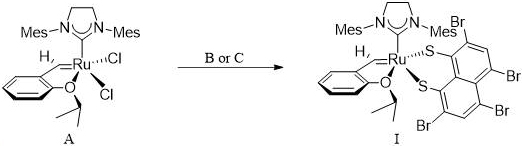
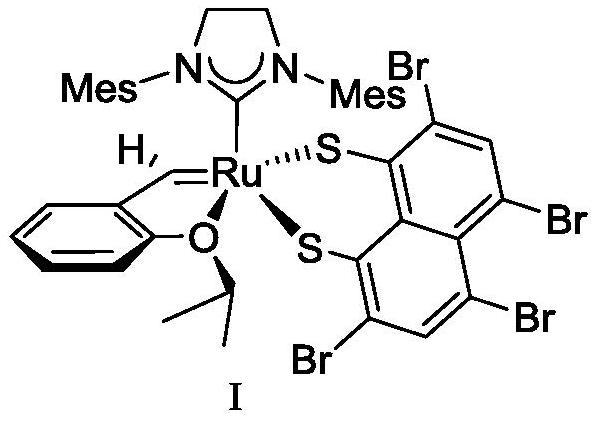
[0036] A Z-selective ruthenium carbene olefin metathesis catalyst has general structural formula I. The preparation process is as follows: under nitrogen protection, in a 10mL round bottom flask, Hoveyda catalyst A (187.5mg, 2.4mmol) and 2,4,5 , 7-Tetrabromo-1,8-dimercapto zinc salt B (236.0mg, 0.4mmol) was dissolved in 5mL tetrahydrofuran, stirred at 0°C for 0.5h, dried in vacuum after the reaction, added dichloromethane and centrifuged to remove After solvent removal, a tan solid powder was obtained, namely the final product I (196.4 mg, yield 74.1%).
[0037]
[0038] 1 H NMR (400MHz, CDCl 3 )δ15.38(s,1H),7.32(d,J=13.6Hz,2H),7.04–6.91(m,3H),6.85–6.67(m,3H),3.99(d,J=7.6Hz,3H ),2.61–2.39(m,9H),2.21(t,J=26.3Hz,9H),1.80(d,J=6.7Hz,4H),1.51(d,J=6.6Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ153.99,142.31,141.42,140.93,135.26,132.32,131.32,131.18,129.50,129.22,127.34,126.40,124.29,124.01,122.69,122.01,115.59,80.77,53.63,51.43,24.33,21.58,21.18,19.23ppm. ESI-MS[M]+calcd for C 41 h ...
Embodiment 2
[0040] The difference between Example 2 and Example 1 is that 2,4,5,7-tetrabromo-1,8-sodium dithioate (C) is replaced by 2,4,5,7-tetrabromo-1,8 -Dimercapto zinc salt (B), the rest were the same as in Example 1 to obtain the final product I (145.6 mg, yield 54.9%).
[0041] 1 H NMR (400MHz, CDCl 3 )δ15.38(s,1H),7.32(d,J=13.6Hz,2H),7.04–6.91(m,3H),6.85–6.67(m,3H),3.99(d,J=7.6Hz,3H ),2.61–2.39(m,9H),2.21(t,J=26.3Hz,9H),1.80(d,J=6.7Hz,4H),1.51(d,J=6.6Hz,3H).13C NMR( 101MHz,CDCl3)δ153.99,142.31,141.42,140.93,135.26,132.32,131.32,131.18,129.50,129.22,127.34,126.40,124.29,124.01,122.69,122.01,115.59,80.77,53.63,51.43,24.33,21.58,21.18, 19.23ppm.
experiment example 1
[0043] Catalytic generation: ((Z)-2-((1’S,3’R)-3’-vinylcyclopentyl)vinyl)benzene
[0044]
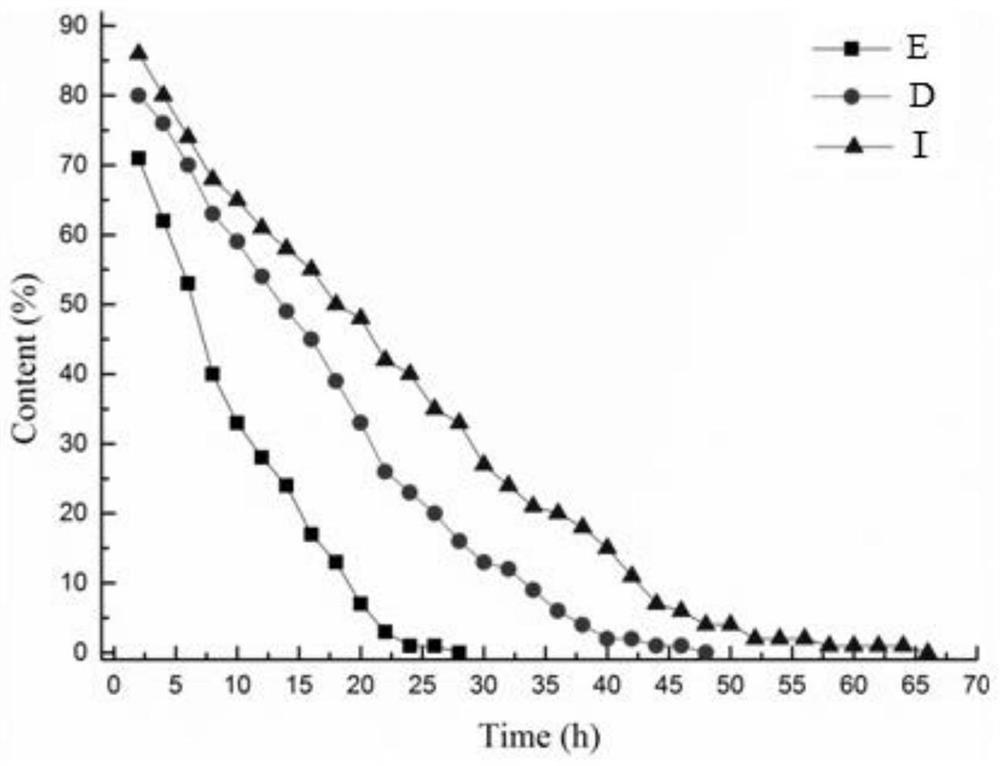
[0045] Under nitrogen protection, 14.3mg (0.15mmol) norbornene and 312.3mg (3mmol) styrene were added in the reaction test tube, followed by adding 1mL THF dissolved in 4.8mg (4.5μmol, 3.0mol%) of the catalyst obtained in Example 1 (tetrahydrofuran) solution, stirred at room temperature for 4h. After the reaction, the product was passed through a silica gel column (10% ethyl acetate in petroleum ether solution-60% ethyl acetate in petroleum ether solution), and finally obtained 28.1 mg of a colorless oil (95.0% yield), Z / E was 98: 2.
[0046] 1 H NMR (600MHz, CDCl 3 ): δ7.36–7.31(m,2H),7.28–7.21(m,3H),6.37(d,J=11.5Hz,1H),5.83(ddd,J=17.4,10.2,7.4Hz,1H), ( m,1H),2.67–2.43(m,1H),2.15–2.00(m,1H),1.97–1.80(m,2H),1.63–1.46(m,2H),1.24(dt,J=12.5,10.4 Hz,1H). 13 C NMR (151MHz, CDCl3) δ143.15, 138.05, 137.92, 128.71, 128.24, 127.72, 126.59, 112.66, 44.65, 41.55, 38.79, 33.13, 32.04ppm. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com