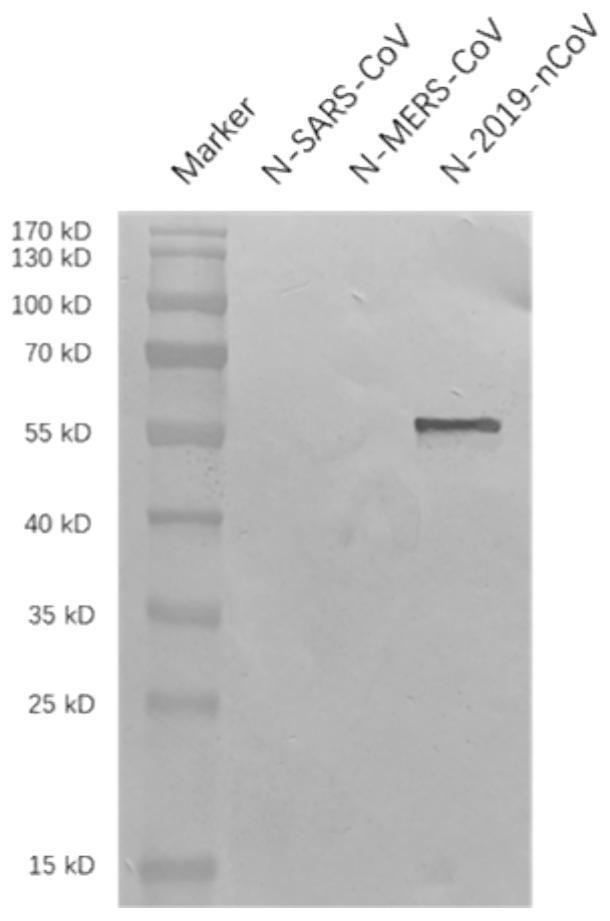
2019-nCoV N protein linear epitope peptide, monoclonal antibody and application
A monoclonal antibody, 2019-ncov technology, applied in the field of immunology, can solve the problem that there is no specific treatment for the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, establishment of hybridoma cell line
[0047] 1. Experimental materials
[0048] 1. Immunogen: In this example, polypeptides 1-5 were coupled to the carrier protein KLH, and the KLH-coupled polypeptides were used as immunogens to immunize animals.
[0049] 2. Experimental animals: BALB / c mice, 4-6 weeks old, female, SPF grade.
[0050] 3. Other materials: Freund's complete adjuvant and Freund's incomplete adjuvant were purchased from Sigma Company; HRP-labeled goat anti-mouse IgG antibody was purchased from JacksonImmune Company; other reagents were domestic analytically pure products.
[0051] 2. Establishment of hybridoma cell lines
[0052] 1. Animal immunization
[0053] 1) Basic immunization: the antigen was mixed with Freund's complete adjuvant in equal volumes and fully emulsified, and injected subcutaneously in points, each injection volume of each Balb / c mouse was 100 μg.
[0054] 2) Booster immunization: The emulsion of antigen and Freund's i...
Embodiment 2
[0063] Example 2 Preparation and evaluation of anti-new coronavirus mouse monoclonal antibody
[0064] 1. Antibody preparation
[0065] Adult BALB / c mice were selected and inoculated intraperitoneally with Freund's incomplete adjuvant, 0.5ml per mouse. After 7-10 days, the 16th generation hybridoma cells were inoculated intraperitoneally, 1×10 per mouse 6 -2×10 6 indivual. After an interval of 5 days, when the abdomen is obviously enlarged and the skin feels tense when touched with hands, the ascites can be collected with a No. 9 needle.
[0066] Centrifuge the ascitic fluid (13000r / min for 30 minutes), remove cell components and other precipitates, and collect the supernatant. Purify with Protein G~Sepharose CL-4B, the upper column liquid is 20mM PBS buffer, and the eluent of column chromatography is: pH 2.7, 20mM glycine buffer, respectively, to obtain the mouse monoclonal anti-new coronavirus N protein Cloned antibodies. Among them, the monoclonal antibody Clone 4G1 w...
Embodiment 3
[0084] Example 3, Application of Purified Antibody Clone 4G1 and Clone 11D5 to Prepare New Coronavirus N Protein Detection Reagent
[0085] Clone 4G1 and Clone 11D5 antibodies were used for paired detection experiments, and Clone4G1 was determined as the capture antibody, and HRP-labeled Clone 11D5 was used as the detection antibody. The ELISA detection method was determined, and the detection sensitivity reached 2.5ng / mL. See Figure 9 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com