Rabbit staphylococcus aureus and application thereof in preparation of inactivated vaccine
A technology for staphylococcus and inactivated vaccine, applied in vaccines, veterinary vaccines, bacteria and other directions, can solve problems such as drug residues, and achieve the effects of simple preparation method, prevention of staphylococcus aureus infecting rabbits, and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
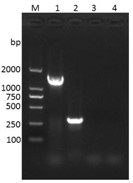
[0017] Isolation, Identification and Screening of Rabbit Staphylococcus Aureus
[0018] 1) Aseptically collect lung samples, mastitis samples, and foot dermatitis samples from dead rabbits, and inoculate each sample with 5% (V / V) defibrillated sheep blood-brain infusion (Oxoid company, article number: CM1135B) agar plate, cultivated at 37°C for 24 hours, pick a round colony with a slight bulge in the center, light yellow, complete hemolysis, and neat edges, on a 5% (V / V) defibrillated sheep blood brain heart infusion agar plate Purified 3 times in succession to obtain a purified culture.
[0019] 2) Take the above-mentioned pure culture, spread it evenly on a glass slide to prepare a smear, and observe the bacterial morphology with a microscope after Gram staining. Select single, paired or clustered Gram-positive cocci for further identification.
[0020] 3) Extract the genomic DNA of the strain screened in step 2) using 16S rRNA with nuc Gene primers were used to amplif...
Embodiment 2
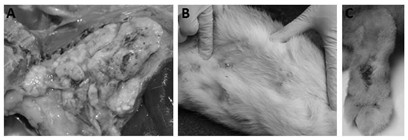
[0024] Preparation and Safety Test of Rabbit Staphylococcus Aureus Inactivated Vaccine
[0025] 1. Preparation of Rabbit Staphylococcus aureus Inactivated Vaccine
[0026] Rabbit Staphylococcus aureus SA472 was inoculated into 500 mL of brain heart infusion medium at a volume ratio of 0.1%, and cultured at 180 rpm at 37°C for 24 hours; added formaldehyde to a final volume concentration of 0.3%, and inactivated at 100 rpm at 37°C for 24 hours to obtain inactivated Live bacteria liquid; add the commercialized MONTANIDE of the French Sepic company to the inactivated bacteria liquid TM GEL 02 PR adjuvant to a final volume concentration of 10%, mixed evenly to obtain an inactivated vaccine, sealed after aliquoting, and stored at 4°C.
[0027] 2. Safety testing of rabbit Staphylococcus aureus inactivated vaccine
[0028] 1) Sterility test: Take 0.2 mL of the inactivated vaccine prepared in step 1, spread evenly on the brain-heart infusion agar plate containing 5% (V / V) defibrinat...
Embodiment 3
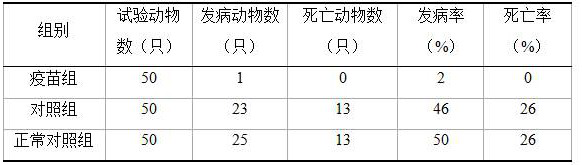
[0033] Evaluation of Immunity Efficacy of Inactivated Vaccines
[0034] 1. The growth and decline of antibodies after inactivated vaccine immunization
[0035] A total of 40 healthy rabbits negative for Staphylococcus aureus and negative for anti-Staphylococcus aureus antibodies were selected and divided into two groups on average. Immune group: 20 experimental rabbits, 10 each of male and female, 1 mL of the vaccine of the present invention was injected subcutaneously at the back of the neck at the age of 35 days, and 1 mL of the vaccine of the present invention was immunized for the second time at the age of 50 days, about 230 days of age (female) Rabbits were immunized with 1 mL of the vaccine of the present invention for the third time; control group: 20 experimental rabbits, 10 male and female, subcutaneously injected 1 mL of sterilized brain-heart infusion medium at the back of the neck at the age of 35 days, and 50 days 1 mL of sterilized brain-heart infusion medium wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com