Degradable monomer based on cyclic acetal structure as well as synthesis method and application of degradable monomer
A cyclic acetal and synthesis method technology, applied in recycling technology, organic chemistry, plastic recycling, etc., can solve the problems of large resin shrinkage rate and high curing temperature, and achieve good controllability, simple synthesis process, and excellent mechanical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Another aspect of the embodiments of the present invention provides a method for synthesizing a degradable monomer based on a cyclic acetal structure, which includes: making a substance containing hydroxybenzaldehyde, The mixed system of glycerol is reacted at 10-180°C to obtain a degradable monomer based on a cyclic acetal structure.
[0034] In some embodiments, the hydroxybenzaldehydes have a structure represented by the following formula:
[0035]
[0036] R 1 , R 2 , R 3 , R 4 , R 5 At least one of them is hydroxyl, at least the other is independently selected from substituted or unsubstituted alkyl, substituted or unsubstituted alkoxy or hydroxyl, and the rest are selected from H, substituted or unsubstituted alkyl, substituted or Unsubstituted alkoxy or hydroxy.
[0037] In some embodiments, the molar ratio of glycerol to hydroxybenzaldehydes is 1:1-10.
[0038] In some embodiments, the catalyst includes any one or a combination of p-toluenesulfonic aci...
Embodiment 1
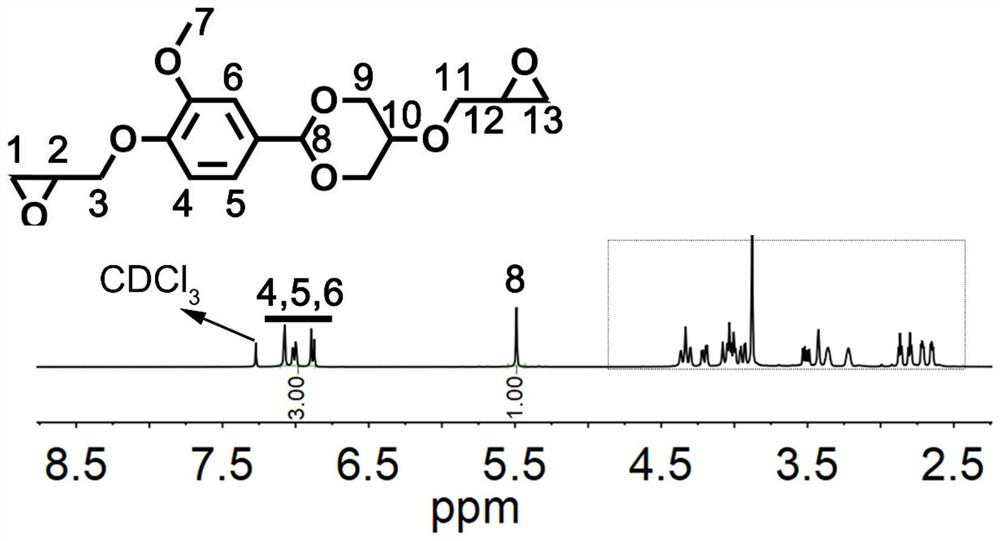
[0069] (1) Take 15.2g of vanillin, 10g of glycerol and 0.15g of phosphoric acid and mix them without adding any solvent, and react at 110°C for 6h until the solid precipitation reaction ends. Wash with a small amount of water and acetone, filter with suction, and dry to obtain the final product. The structural formula is shown in the following formula I-1, and the yield is 90%. 1 H-NMR such as figure 1 As shown, there is a one-to-one correspondence between each peak on the figure and the hydrogen above the structure of formula I-1.
[0070]
[0071] (2) Take 10 g of the compound represented by formula I-1, 0.1 g of tetrabutylammonium bromide, and 25 g of 50 wt % potassium hydroxide aqueous solution, and react at 60° C. for 2 h. Then the temperature was lowered to below 10°C, and 50 g of epichlorohydrin was added dropwise through a constant pressure separating funnel, and the drop was completed within 0.5 h. After the dropwise addition was completed, the reaction was carr...
Embodiment 2
[0074] (1) Mix 15.2g of 2,4-dihydroxy-3-methylbenzaldehyde, 10g of glycerin and 0.15g of citric acid, add 30ml of N,N-dimethylformamide and 40ml of petroleum ether, and react at 100°C for 12h , until there is no more water in the water separator, the reaction is over. Then remove the upper layer of petroleum ether, wash with a small amount of water and acetone, filter with suction, and dry to obtain the final product. H NMR 1 H-NMR and infrared characterization confirmed that the structural formula of the final product was shown in the following formula I-2, and the yield was 80%.
[0075]
[0076] (2) Take 10 g of the compound represented by formula I-2, 10 g of tetrabutylammonium bromide, and 500 g of 50 wt % potassium hydroxide aqueous solution, and react at 10° C. for 2 h. Then the temperature was lowered to -5°C, and 500 g of epichlorohydrin was added dropwise through a constant pressure separating funnel, and the drop was completed within 5 hours. After the dropwis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Pencil hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com