Anti-osteoporosis acacetin derivative and preparation method thereof
An anti-osteoporosis and acacetin technology, which is applied in bone diseases, drug combinations, organic chemistry, etc., can solve the problems of decreased oral bioavailability, poor solubility of acacetin, and inability to fully dissolve, so as to be beneficial to osteoporosis. The deposition, post-processing is simple, the effect of environmental pollution is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
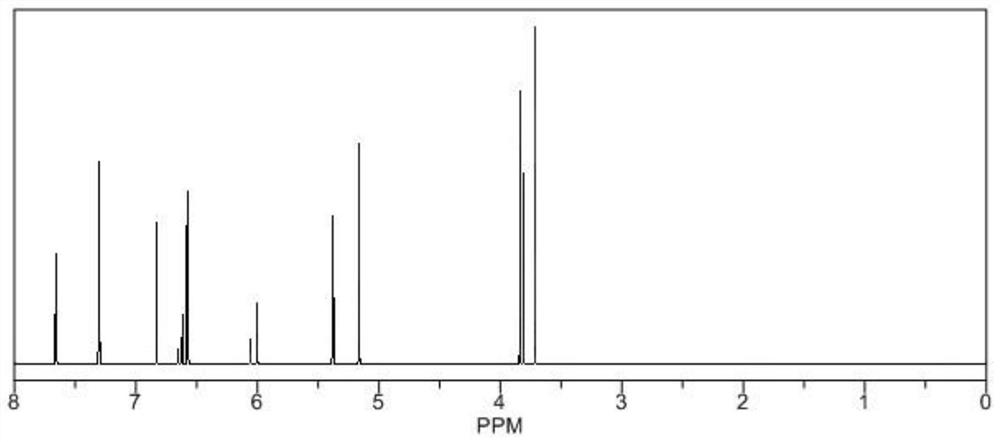
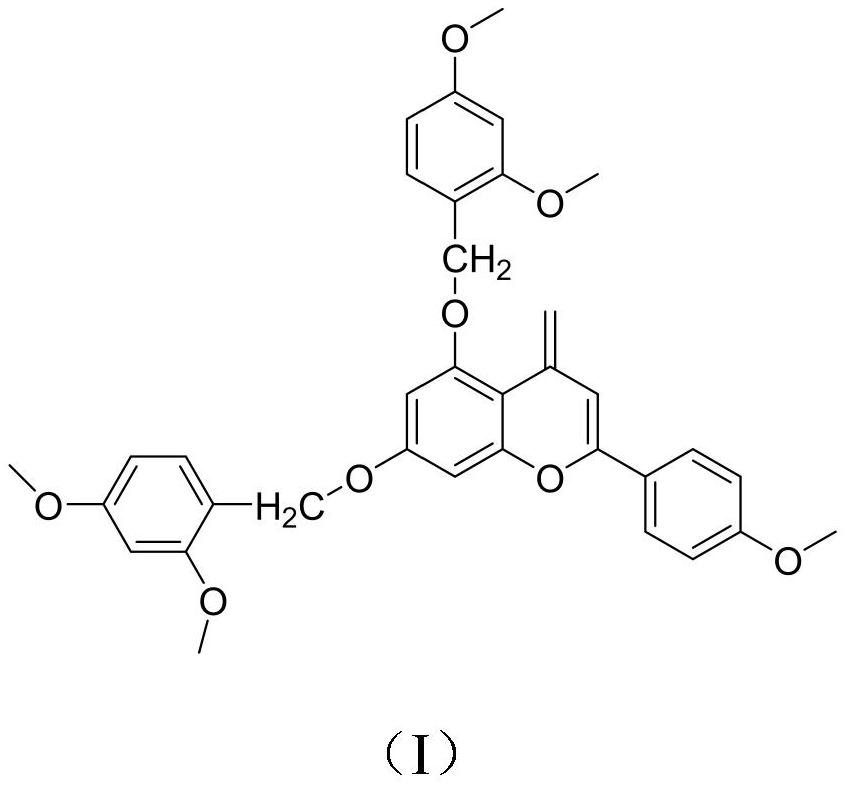
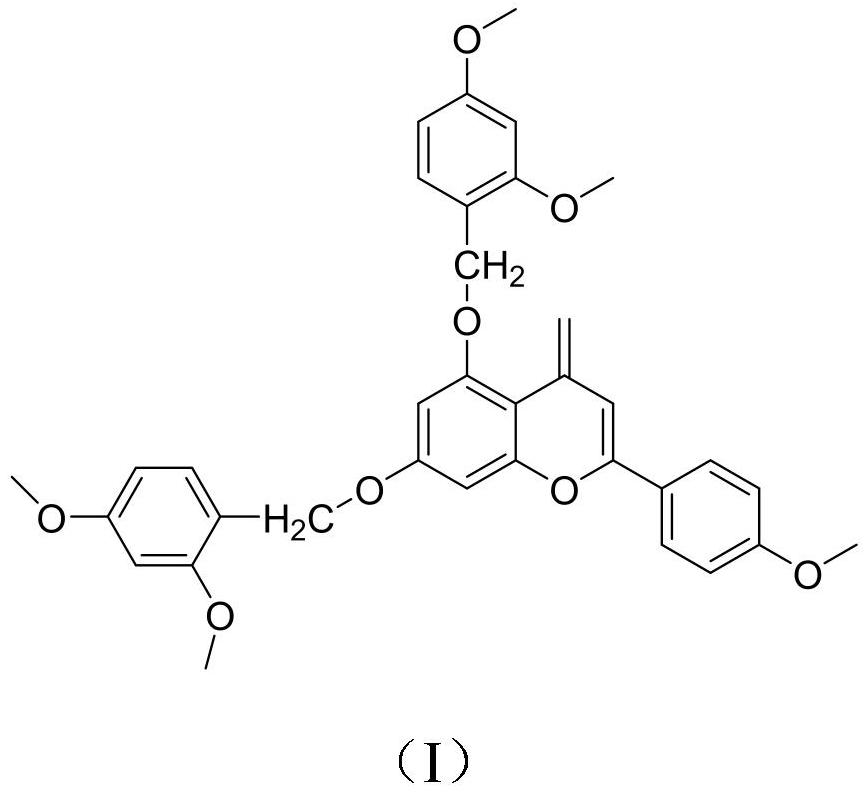
[0018] Under nitrogen protection, dissolve 15g acacetin and 14.8g 2,4-dimethoxybenzyl chloride in 78mL of anhydrous dichloromethane, raise the temperature to 110-120°C, and reflux under the action of triethylamine After 8 hours of reaction, cool to room temperature, add 30-40 mL of ethyl acetate to the reaction liquid for extraction and separation, combine the organic phases, dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain crude products that are separated by silica gel column chromatography , using petroleum ether-ethyl acetate for gradient elution, the volume ratio of petroleum ether to ethyl acetate is 60:1, 40:1, 20:1, 10:1, 5:1, 1:1, TLC detection, The same fractions were combined to obtain a total of 10 fractions, and the fourth fraction was subjected to silica gel column chromatography, using petroleum ether-ethyl acetate 10:1, 5:1 gradient elution, and the collected eluent was recrystallized by ethanol obtain the...
Embodiment 2
[0021] The water solubility test of embodiment 2 compounds of the present invention
[0022] Weigh 0.1 g of the acacetin derivative obtained in Example 1 into a test tube, add 10.0 mL of purified water, shake for 30 seconds every 5 minutes at room temperature, observe the dissolution after 30 minutes, record the amount of solvent, and convert the experimental results into standard solubility (25°C), the test results are shown in Table 1.
[0023] Table 1 Example 1 compound solubility and yield
[0024]
[0025] The results show that, compared with acacetin, the water solubility of the acacetin derivatives is significantly improved, thus indicating that the method of the invention can obtain the acacetin derivatives with better water solubility.
experiment example 3
[0026] Experimental Example 3 Pharmaceutical Research on the Anti-osteoporosis of Compounds of the Present Invention
[0027] Experimental method: select healthy 5-month-old female SD rats, body weight 280 ± 50g, divide the rats into random groups, and divide them into normal control group (control group 1), ovariectomized model group (control group 2), acacetin group ( Control group 3), acacetin derivatives (experimental group), each group of rats was cultivated for 2 weeks in a suitable environment, and each group of female experimental rats was injected with 2% pentobarbital intraperitoneally at a dose of 40 mg / kg body weight After sodium anesthesia, the abdominal cavity of the normal control group was opened and then sutured. The rats in the other groups underwent bilateral ovariectomy. From the third week after the operation, the rats in each group were fed with feed and water every day. Rats in the derivative group were given acacetin and acacetin derivatives at a dose o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com