A purification method for reducing protein content in sodium hyaluronate and its derivatives
A protein content, sodium hyaluronate technology, applied in the field of pharmaceutical production, can solve the problems of low protein content sodium hyaluronate and its derivatives, failure to meet the quality standards of sodium hyaluronate, waste of time and energy consumption, etc., to achieve easy operation of the process , reduce the risk of allergic reactions, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
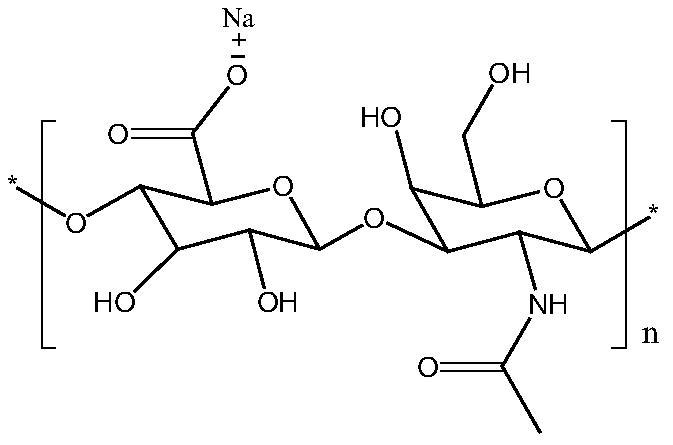
[0036]The preparation method of directly extracting sodium hyaluronate from chicken comb is prepared with reference to the method of patent CN 104610466A. The brief steps are as follows: the thickness of the cockscomb is finely washed again, the cockscomb is minced and then enzymatically hydrolyzed, and the enzymatic hydrolysis solution is obtained. The protein content of sodium hyaluronate obtained by direct alcohol precipitation of the cockscomb enzymolysis solution was 1.2% by the Folin phenol reagent method. The protein content was too high and did not meet the quality standard. Examples are experiments after treatment with protein complexing agents.
[0037] The sodium hyaluronate prepared by the microbial fermentation method is a commercially available product, and the protein content determined by the Folin phenol reagent method is 0.08%.
Embodiment 1
[0038] Example 1: Refinement of cross-linked sodium hyaluronate
[0039] Take 1 L of cockscomb extract, add 0.2 g of inositol hexaphosphate (50% aqueous solution) under stirring, filter through a 0.22 μm microporous membrane after stirring for 24 hours, add 2 L of ethanol to the filtrate, and after the addition is completed, a white solid is formed Precipitation, suction filtration, dehydration for three times and drying to obtain cross-linked sodium hyaluronate with a protein content of 0.011%.
Embodiment 2
[0040] Example 2: Refinement of cross-linked sodium hyaluronate
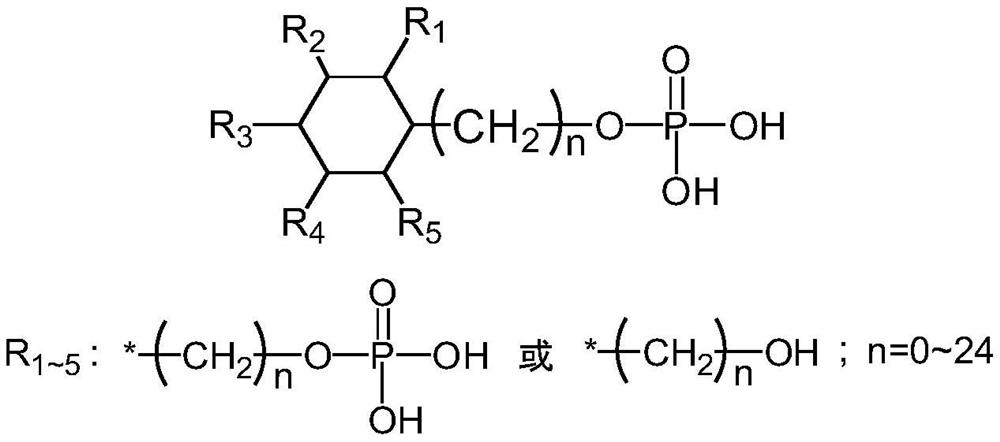
[0041] Take 1 L of cockscomb extract, add 1 g of CPPM (CAS No.2271351-38-1, 1,2,3,4,5-Cyclohexanepentol,6-[(phosphonooxy)methyl]) under stirring, stir for 0.5 hours and pass through 0.22 Filtration with a μm microporous membrane, adding 2L of ethanol to the filtrate, after the addition was completed, a white solid was precipitated after stirring, suction filtration, dehydration three times, and drying to obtain cross-linked sodium hyaluronate with a protein content of 0.013%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com