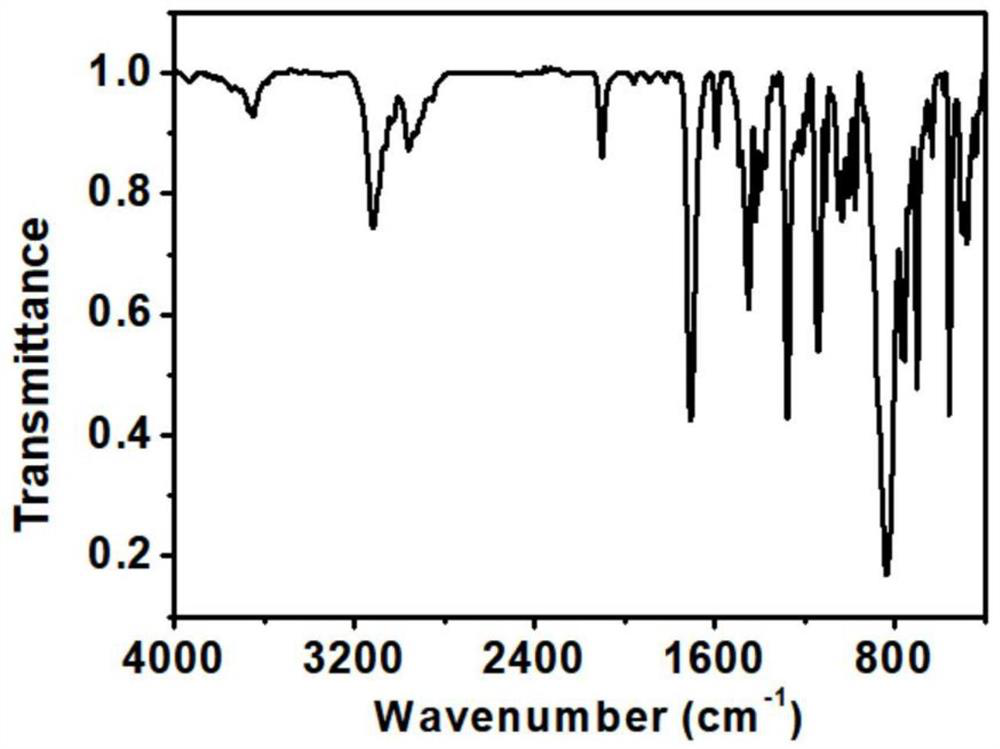
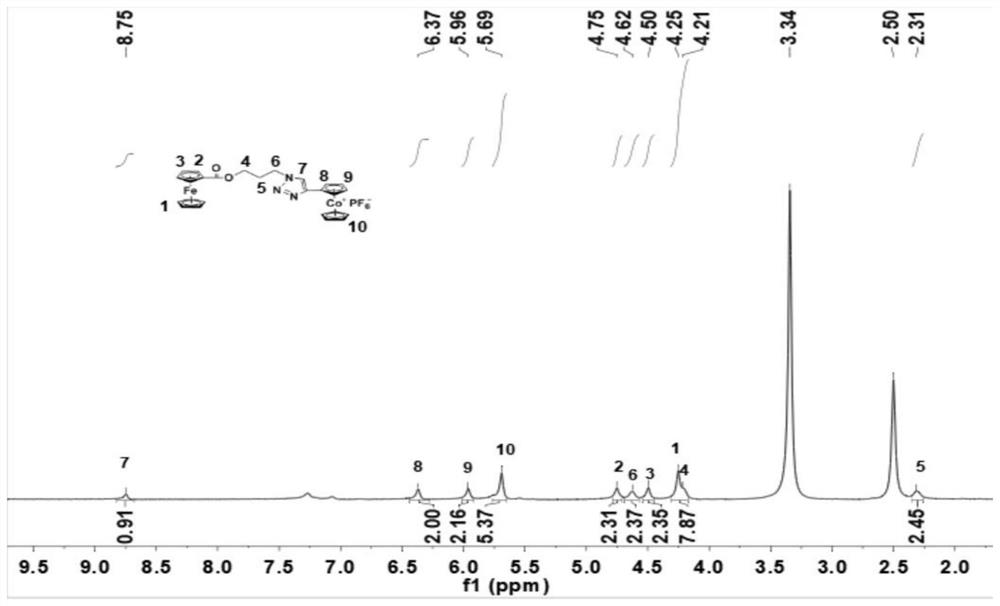
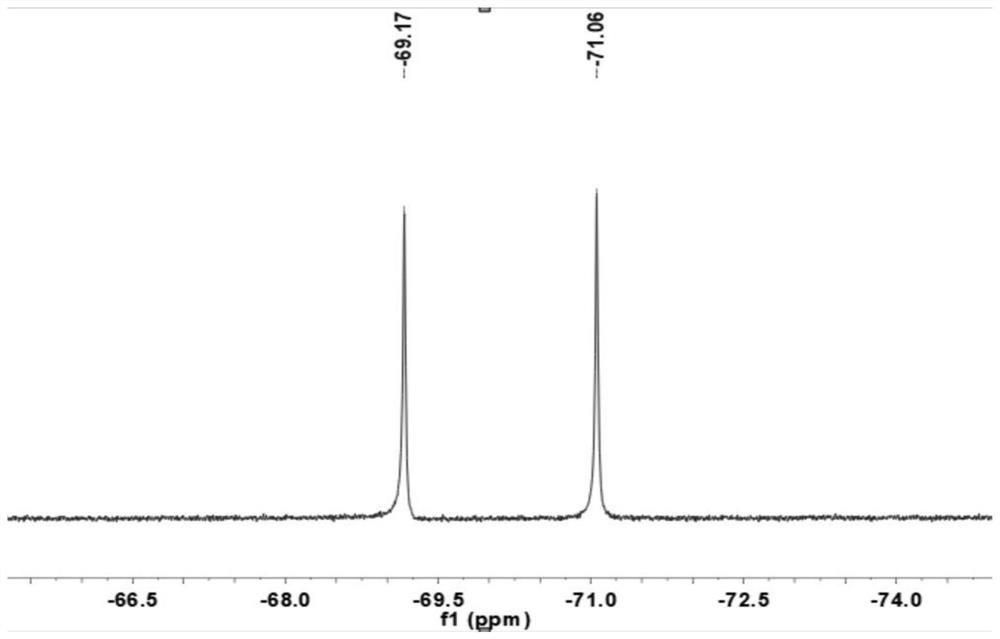
Asymmetric cobaltocene cation derivative and preparation method thereof
A cobaltocene cation and asymmetric technology, applied in the field of asymmetric cobaltocene cation derivatives and their preparation, can solve the problem of less asymmetric cobaltene cation derivatives and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention provides the preparation method of the asymmetric cobaltocene cation derivative described in the above technical scheme, comprising the following steps:
[0050] when R is -C 7 H 9 COO(CH 2 ) 3 or R for and R' is -C 7 H 9 COOCH(CH 2 ) 2 or R for and R" is -C 7 H 9 COOCH 2 C(CH 2 ) 3 Time:
[0051] when X is PF 6 , the preparation method of the asymmetric cobaltocene cation derivative comprises the following steps:
[0052] The azido-alcohol compound, trimethylsilylethynyl cobaltocene hexafluorophosphate, catalyst, potassium carbonate and the first solvent are mixed, and a first click reaction is carried out to obtain a click product; the azido- The alcohol compound is 3-azido-1-propanol, 1,3-diazido-2-propanol or triazido-neopentanol;
[0053] Mixing the click product, 4-dimethylaminopyridine, norbornenecarbonyl chloride and the second solvent, and performing alcoholysis reaction to obtain an asymmetric cobaltocene cation derivat...
Embodiment 1
[0098]When R=-C 7 h 9 COO(CH 2 ) 3 Time:
[0099] Add 3-bromo-1-propanol (1.112g, 8mmol) into 10mL of anhydrous N,N-dimethylformamide (DMF), stir to dissolve, add sodium azide (1.56g, 24mmol), and After the reaction was completed and cooled to room temperature, DMF was distilled off under reduced pressure; 10 mL of dichloromethane and 10 mL of deionized water were added to the distilled mixture, and after the solid was completely dissolved, it was transferred to a separatory funnel, and 20 mL of Dichloromethane, extract and separate liquid, wash the organic phase three times with deionized water, then back-extract the aqueous phase with dichloromethane, combine the organic phases; add anhydrous sodium sulfate to the obtained organic phase, filter and wash the filter cake with dichloromethane To colorless, the filtrates were combined, the solvent was removed by rotary evaporation, and dried in vacuo to obtain 3-azido-1-propanol;
[0100] Take a 100mL round bottom flask, di...
Embodiment 2
[0106] When R=-C 5 h 4 C 5 h 5 FeCOO (CH 2 ) 3 Time:
[0107] Add 3-bromo-1-propanol (1.09g, 7.83mmol) and 4-dimethylaminopyridine (0.957g, 7.83mmol) into a 100mL round bottom flask, dissolve with 20mL of anhydrous dichloromethane, and Stir for 30min, then dissolve ferrocenecarbonyl chloride (1.08g, 4.35mmol) in 15mL of anhydrous dichloromethane and transfer it to a constant pressure dropping funnel, and slowly drop it into the above-mentioned round-bottomed flask under ice-bath conditions, After the reaction was over, add 50 mL of distilled water to quench the reaction, and extract the solution with dichloromethane, then back-extract the aqueous phase three times with dichloromethane; combine the organic phases, dry with anhydrous sodium sulfate, and rotary evaporate to obtain the crude product, column chromatography Purification to obtain ferrocenecarboxylic acid-3-bromo-1-propyl ester;
[0108] Add the ferrocenecarboxylic acid-3-bromo-1-propyl ester (0.98g, 2.79mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com