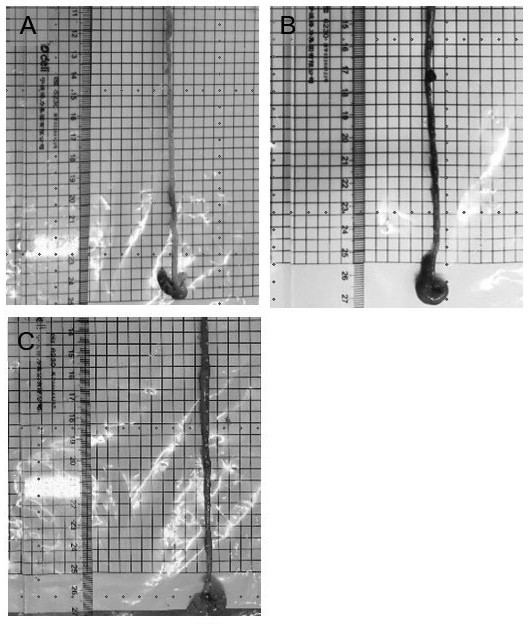
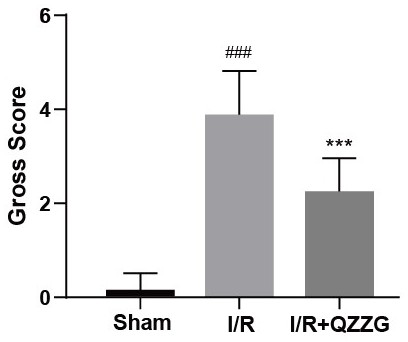
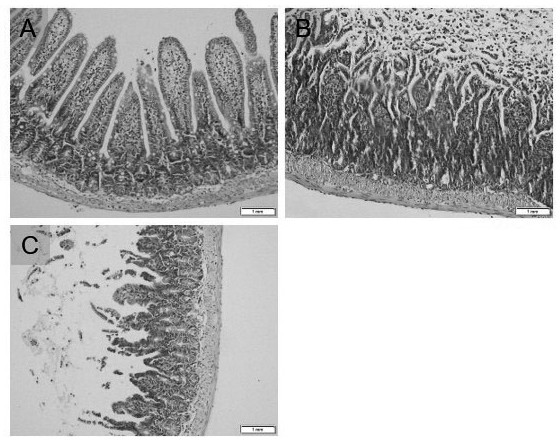
Application of piceatannol-3'-O-beta-D-glucopyranoside in treatment of small intestine ischemia/reperfusion injury
A technology of ischemia-reperfusion and tristilbene, which is applied to the application field of tristilbene in the treatment of small intestine ischemia-reperfusion injury, and can solve the problems of unsatisfactory effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The therapeutic use of the present invention is proved by the following experiments:
[0021] Materials and methods
[0022] Drugs and Reagents
[0023] Tristilbene was extracted from Rheum officinalis in Lhasa, and the purity was higher than 98.0%. TNF-α ELIASA kit was purchased from Shanghai Jianglai Biotechnology Co., Ltd. (Shanghai, China); eNOS ELIASA kit and T-SOD biochemical kit were purchased from Elabscience Company (Wuhan, China). MPO and MDA biochemical kits were purchased from Nanjing Jiancheng Biological Co., Ltd. (Nanjing, China).
[0024] experimental animals
[0025] Male Kunming mice, (25g±2g, Liaoning Changsheng Biotechnology Co., Ltd., Benxi, China). Raised in a constant temperature (20±2°C) environment, 12h cycle light, fed with regular diet and water. The experimental protocol was approved by the Animal Experiment Ethics Committee of Dalian Medical University (ethics number: AEE20024).
[0026] surgical procedure
[0027] The mice were fasted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com