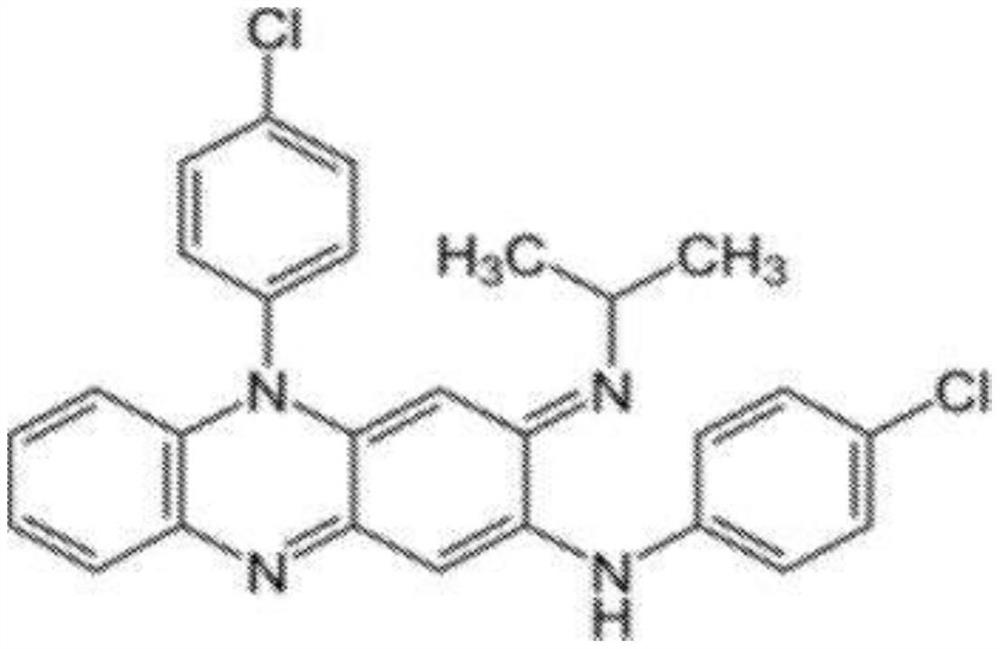
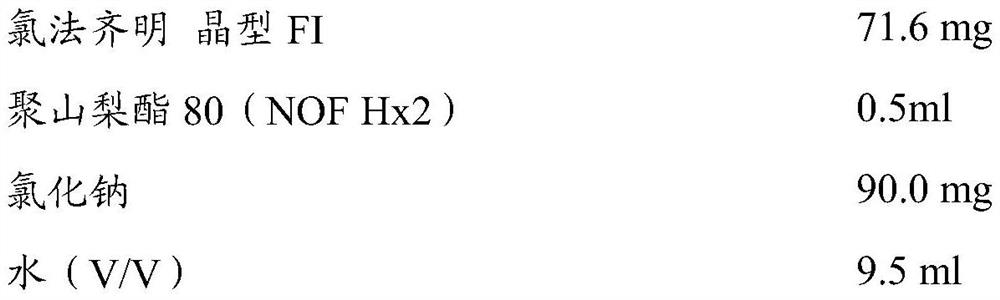Compositions of clofazimine, combinations comprising them, processes for their preparation, uses and methods comprising them
A technology of clofazimine and its composition, applied to the composition of clofazimine, can solve the problems of high mortality, poor diagnosis, limited treatment options, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] 200 mg of clofazimine (as triclinic form I), 90 mg of sodium chloride and 9.5 ml of water were mixed twice in an Ultra-Turrax homogenizer at a speed of 10000 rpm for 5 minutes each. 0.5 ml of polysorbate 80 (NOF Hx2) was added. Using an ultrasound probe (Branson Digital Sonifier TM 250D with Bandelin Sonoplus Probe MS73) to process the mixture seven times, 3 minutes each time, with an amplitude of 70%. The volume was adjusted to 10 ml with water. The suspension was filtered through VWR folded qualitative filter paper (303, particle retention 5-13 μm, size: 150 mm) to obtain the composition of Example 1. The median particle diameter of clofazimine in the composition of Example 1 was 3.9 μm, and the D90 was 6.7 μm. The concentration of clofazimine was determined by 280nm ultraviolet / visible spectrophotometry, calibrated with 1 mg / ml clofazimine stock solution (stock) diluted in mobile phase, and the measurement result was 7.16 mg / ml.
[0193] Compositions in Example ...
Embodiment 2
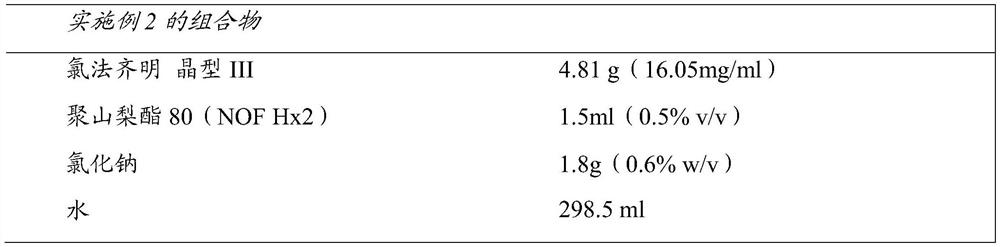
[0199] In 100 ml of water containing 0.5% polysorbate 80 (NOF Hx2) and 0.6% sodium chloride, 6 g of orthorhombic form III clofazimine was added, and pre-micronized using an Ultra-Turrax at 10,000 rpm for about 40 seconds. Preformulations were prepared by adding 0.6% sodium chloride in water to give a volume of 300ml. 300 ml of this suspension was added to the inlet of a homogenizer (M-110EH-30 Microfluidizer (Microfluidics, Westwood, MA, USA)) and pre-homogenized for 15 minutes by circulating the suspension through the H30Z chamber at 5000 psi. qualitative step. Subsequently, a second H10Z chamber was installed in series with the first chamber, and the suspension was further homogenized at 25,000 psi for 23 minutes. Particle size analysis using HORIBA LA 950 showed a median particle size of 0.83 μm and a D90 value of 1.17 μm. The 1 mg / ml clofazimine stock solution diluted in the mobile phase was used for calibration, and the concentration of clofazimine was determined to be ...
Embodiment 3
[0204] Suspensions of clofazimine (crystal-modified orthorhombic form III) in water, sodium chloride, and polysorbate 80 solutions were treated using an M-110EH-30 microfluidizer processor (chambers: H30Z and G10Z), The composition of Example 3 was produced in the H30Z-G10Z configuration at a pressure of 28250 psi for 30 minutes, resulting in clofazimine particles with a median particle size of 1.28 μm and a D90 of less than 2 μm.
[0205] Compositions in Example 3 are shown in Table 3
[0206]
[0207] table 3
[0208] Viscosity measurement
[0209] The viscosity of the composition of Example 3 was tested using a STRESSTECH rheometer in stress control mode. A double gap geometry is employed and the spindle rotates continuously to ensure that the particles remain suspended during the temperature point. Viscosities measured at 20°C, 25°C, and 30°C across stresses of 0.01, 0.05, and 0.1 Pa, respectively. Two separate loadings were performed to obtain the average viscosi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com