Preparation method of EDDHA chelated ferric salt
A technology for chelating iron and salt solution is applied in the field of preparation of EDDHA chelated iron salt, and can solve the problems affecting the purity of EDDHA chelated iron salt, large impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
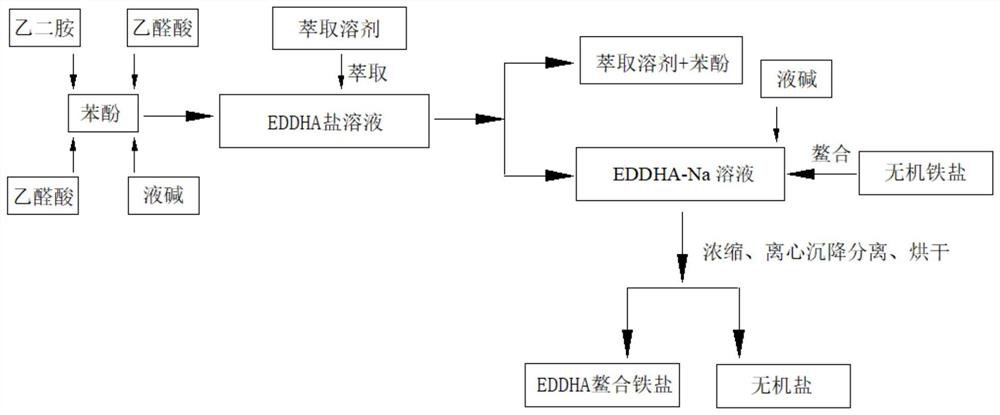
[0029] A preparation method of EDDHA chelated iron salt, the concrete processing steps are:
[0030] Add 564.66g of phenol into a 2000ml four-necked bottle, stir and heat to 40°C, start to drop 71.07g of 50wt% glyoxylic acid and 36g of ethylenediamine at the same time at a constant speed, and the dropping time is 1.5h. After the dropwise addition, 71.07g of 50wt% glyoxylic acid and 75g of 32% lye (sodium hydroxide solution) were added dropwise at a constant speed for 1.5 hours. After the dropwise addition was completed, the temperature was raised to 60° C. and kept for 5 hours to obtain the reaction product. The temperature of the reaction product was lowered to 42° C., 540 g of water was added, and then 67.89 g of toluene was added to extract the reaction product, stirred for 10 min, and separated after standing for 10 min to obtain an aqueous phase. This extraction process was repeated twice (33.95 g of toluene each time), and the aqueous phases obtained by the three extrac...
Embodiment 2
[0034] A preparation method of EDDHA chelated iron salt, the concrete processing steps are:
[0035] Add 847g of phenol into a 2000ml four-neck flask, stir and heat to 50°C, start to drop 88.84g of 50wt% glyoxylic acid and 36g of ethylenediamine at the same time at a constant speed, and the dropping time is 1.5h. After the dropwise addition, 88.84g of 50wt% glyoxylic acid and 76.5g of 32% lye (sodium hydroxide solution) were added dropwise at a constant speed for 1.5 hours. After the dropwise addition was completed, the temperature was raised to 75° C. and kept for 3 hours to obtain the reaction product. The temperature of the reaction product was lowered to 42° C., 540 g of water was added, and 167.72 g of butyl acetate was added to extract the reaction product, stirred for 15 min, left to stand for 15 min, and separated to obtain an aqueous phase. The extraction process was repeated twice more (the amount of butyl acetate was 86.86g), and the aqueous phases obtained by the ...
Embodiment 3
[0039]A preparation method of EDDHA chelated iron salt, the concrete processing steps are:
[0040] Add 1242g of phenol into a 2000ml four-necked bottle, stir and heat to 70°C, and start to dropwise add 106.6g of 50wt% glyoxylic acid and 36g of ethylenediamine simultaneously at a constant speed for 1.5h. After the dropwise addition, 106.6g of 50wt% glyoxylic acid and 78.75g of 32% lye (sodium hydroxide solution) were added dropwise at a constant speed for 1.5 hours. After the dropwise addition was completed, the temperature was raised to 90° C. and kept for 1 hour to obtain the reaction product. The temperature of the reaction product was lowered to 42° C., 540 g of water was added, and 316.5 g of carbon tetrachloride was added to extract the reaction product, stirred for 20 min, and separated after standing for 20 min to obtain an aqueous phase. This extraction process was repeated twice (the amount of carbon tetrachloride each time was 158.25g), and the aqueous phases obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com