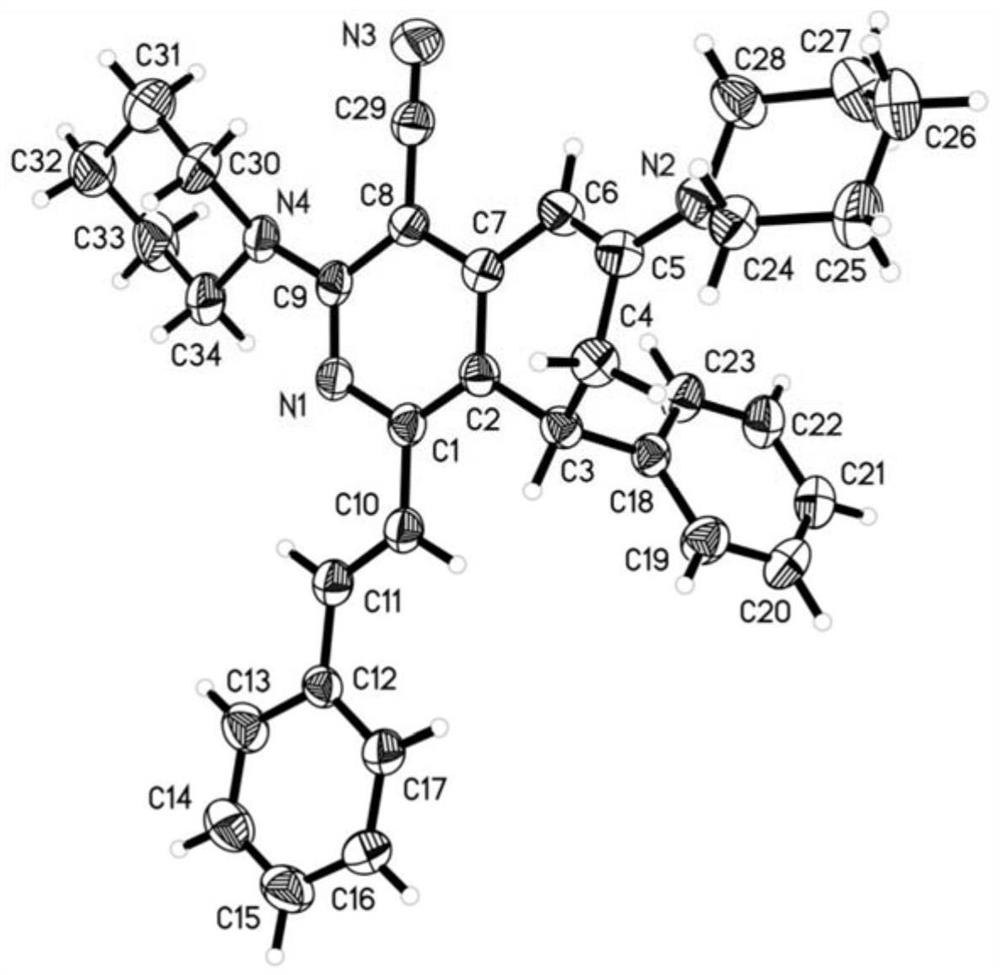
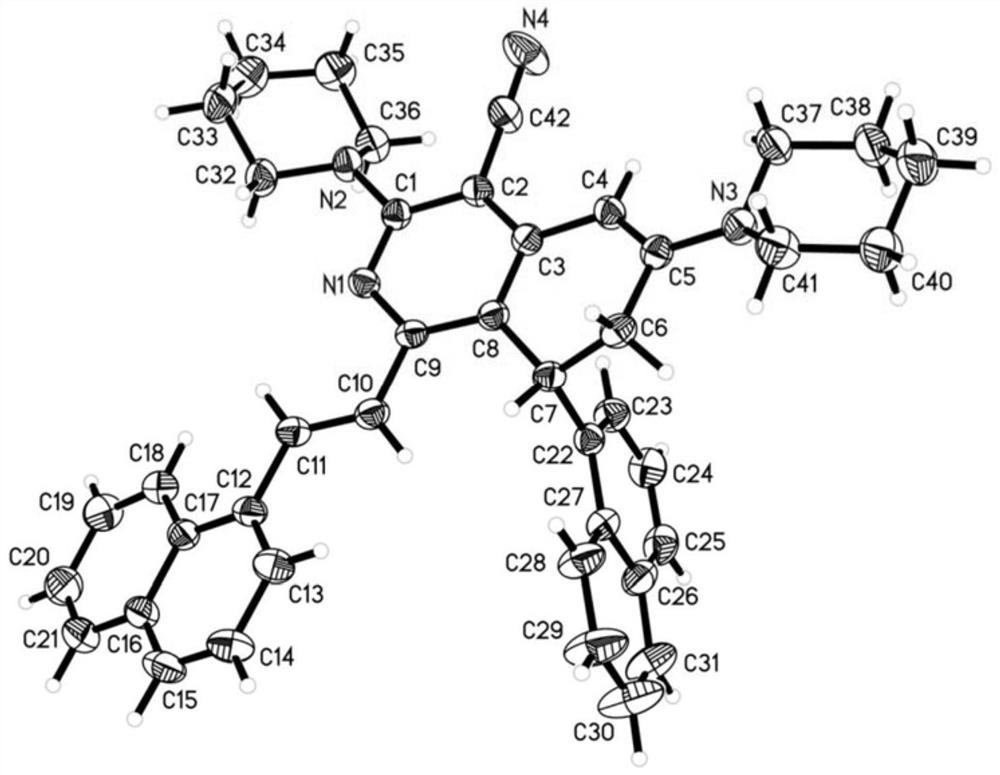
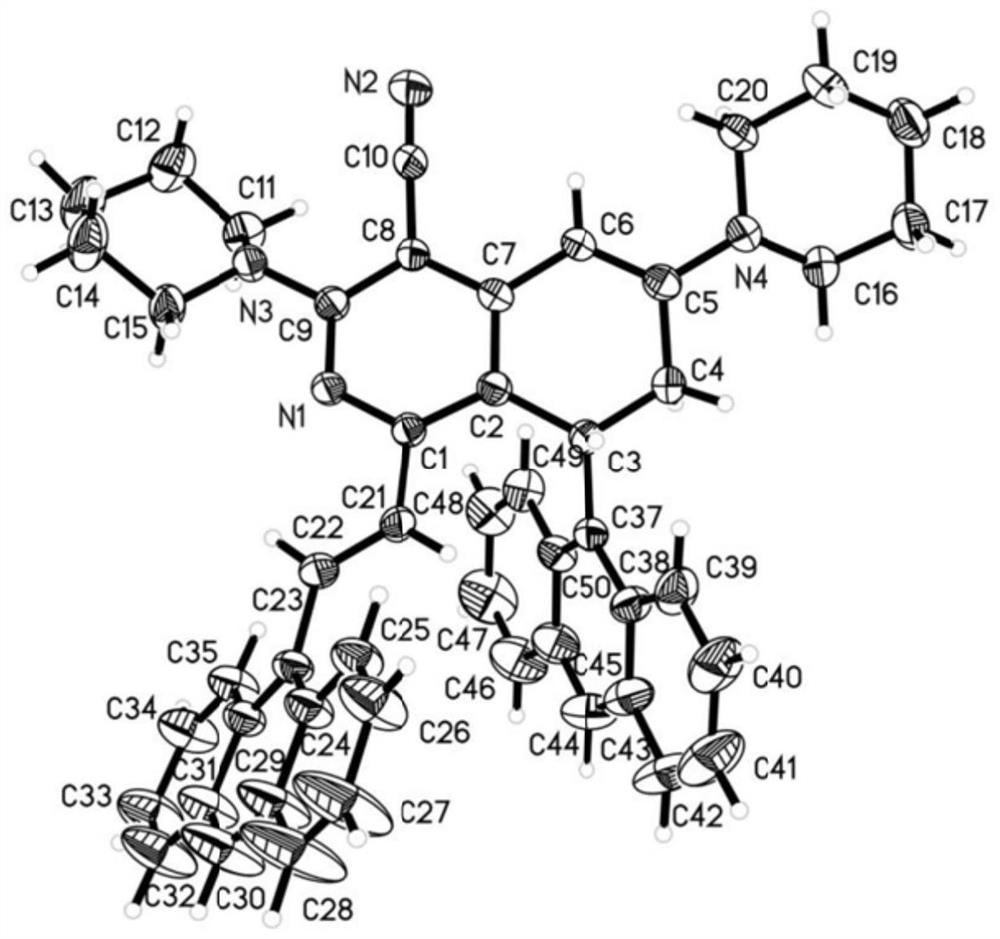
4-cyano-7, 8-dihydroisoquinoline derivative as well as preparation method and application thereof
A technology of dihydroisoquinoline and derivatives, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of expensive use, complicated steps, toxic metal catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] This example provides 4-cyano-7,8-dihydroisoquinoline derivative 3aa and its preparation method. The synthesis route is as follows:
[0074]
[0075] Concrete synthetic steps include:
[0076] Take compound 1a 0.3mmol, secondary amine 2a 1.2mmol and sodium acetate 0.6mmol, join in 3mL of DMSO, at 120 ℃ and N 2 The reaction was stirred under atmosphere for 14 h; after the reaction was completed, after the reaction liquid was cooled to room temperature, 20 mL of CH 2 Cl 2 , with water and CH 2 Cl 2 After extraction three times, the organic phase was collected and dried with anhydrous sodium sulfate, the organic solvent was removed by rotary evaporation under reduced pressure, and the compound was purified by column chromatography to obtain the corresponding compound 3aa.
[0077] Further optimize the reaction conditions, use the above compound 1a and secondary amine 2a to prepare 3aa as a template reaction, explore the reaction conditions such as base, solvent, rea...
Embodiment 2
[0083] This example provides 4-cyano-7,8-dihydroisoquinoline derivatives 3aa-3an and their preparation methods. The synthetic route is as follows:
[0084]
[0085] The specific synthetic steps include: take compound 1a 0.3mmol, secondary amine (2a-2n) 1.2mmol and KH 2 PO 4 0.9mmol, added to 2.5mL of DMSO, at 120°C and N 2 The reaction was stirred under atmosphere for 14 h; after the reaction was completed, after the reaction liquid was cooled to room temperature, 20 mL of CH 2 Cl2 , with water and CH 2 Cl 2 After extraction three times, the organic phase was collected and dried with anhydrous sodium sulfate, the organic solvent was removed by rotary evaporation under reduced pressure, and the compound was purified by column chromatography to obtain the corresponding compound 3aa-3an.
[0086] Wherein, the eluents when the compounds 3aa-3an (except 3ad and 3ag) are purified by column chromatography are as follows:
[0087] 3aa, 3ab, 3ac, 3ae, 3aj, 3al, 3an—petroleum et...
Embodiment 3
[0108] This example provides 4-cyano-7,8-dihydroisoquinoline derivative 3ba-3na and its preparation method. The synthetic route is as follows:
[0109]
[0110] The specific synthetic steps include: take compound 1b-1n 0.3mmol, secondary amine 2a 1.2mmol and KH 2 PO 4 0.9mmol, added to 2.5mL of DMSO, at 120°C and N 2 The reaction was stirred under atmosphere for 14 h; after the reaction was completed, after the reaction liquid was cooled to room temperature, 20 mL of CH 2 Cl 2 , with water and CH 2 Cl 2 After extraction three times, the organic phase was collected and dried with anhydrous sodium sulfate, the organic solvent was removed by rotary evaporation under reduced pressure, and the compound was purified by column chromatography to obtain the corresponding compound 3ba-3bn.
[0111] Wherein, the eluents when the compounds 3ba-3bn are purified by column chromatography are as follows: 3ba, 3ca, 3da, 3ea, 3fa, 3ga, 3ha, 3ia, 3ja, 3ka—petroleum ether with a volume ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com