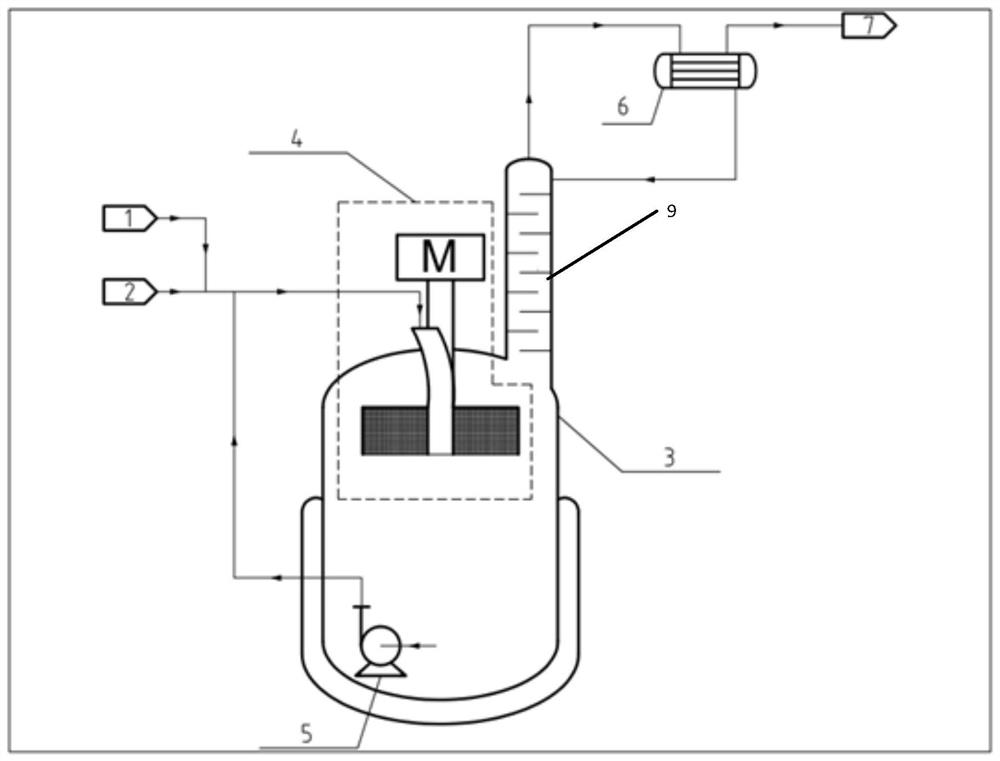
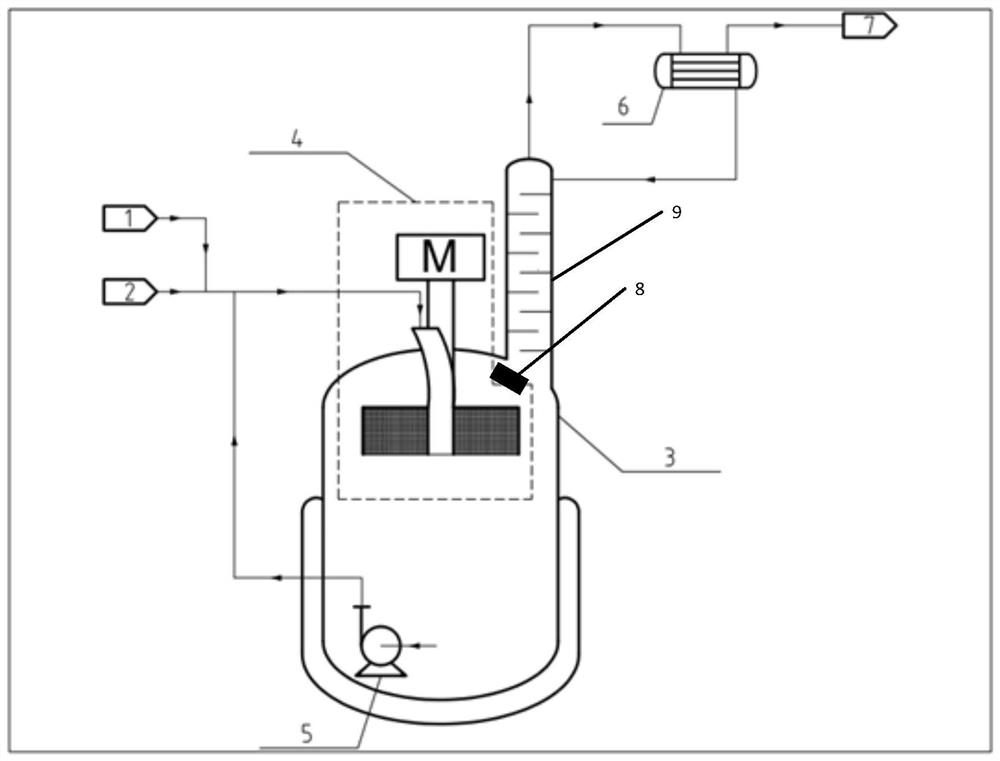
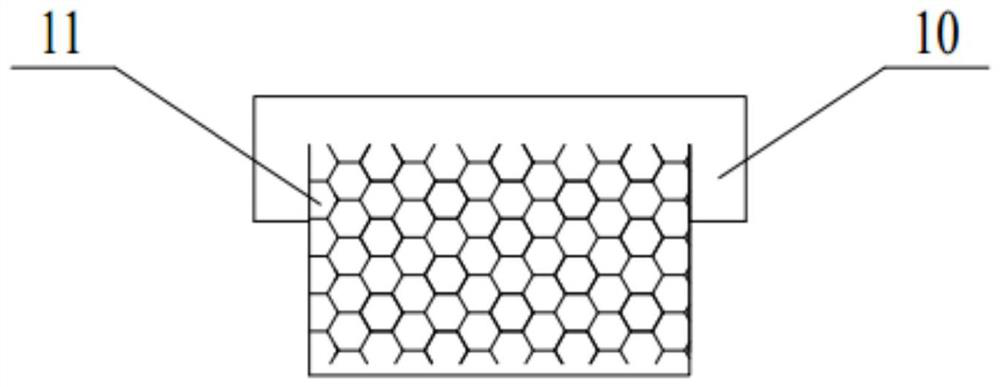
System, method and reaction device for process for preparing trifluoroethane through continuous reaction
A reaction device, the technology of trifluoroethane, applied in the field of chemical engineering, can solve the problems of affecting the main reaction, affecting the utilization rate of raw materials, self-polymerization side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Adopt device and method of the present invention to prepare 1,1,1-trifluoroethane (HFC-143a) by continuous reaction, adopt SnCl Catalyst, the molar ratio of catalyst molecule and the vinylidene chloride substance of fresh feed is 0.2:1 wherein hydrogen fluoride and 1,1,1-trifluoroethane (HFC-143a) feed molar flow ratio is 3:1, the reaction temperature is 85 ℃, the system pressure is maintained at 1.2MPa, the supergravity level of the built-in supergravity reactor is 500g, the circulation ratio is 8:1. It was measured that the conversion rate of vinylidene chloride was 99.3%, and the mass fraction of HFC-143a (without hydrogen chloride) in the outlet gas was 99.8%.
Embodiment 2
[0098] Adopt device and method of the present invention to prepare 1,1,1-trifluoroethane (HFC-143a) by continuous reaction, adopt SnCl Catalyst, the molar ratio of catalyst molecule and the vinylidene chloride substance of fresh feed is 0.2:1 wherein hydrogen fluoride and 1,1,1-trifluoroethane (HFC-143a) feed molar flow ratio is 4:1, the reaction temperature is 85 ° C, the system pressure is maintained at 1.2MPa, the supergravity level of the built-in supergravity reactor is 600g, the circulation ratio is 10:1. It was measured that the conversion rate of vinylidene chloride was 99.8%, and the mass fraction of HFC-143a (without hydrogen chloride) in the outlet gas was 99.9%.
Embodiment 3
[0100] Adopt device and method of the present invention to prepare 1,1,1-trifluoroethane (HFC-143a) by continuous reaction, adopt SnCl Catalyst, the molar ratio of catalyst molecule and the vinylidene chloride substance of fresh feed is 0.2:1 wherein hydrogen fluoride and 1,1,1-trifluoroethane (HFC-143a) feed molar flow ratio is 3:1, the reaction temperature is 80 ℃, the system pressure is maintained at 1.2MPa, the supergravity level of the built-in supergravity reactor is 300g, the circulation ratio is 8:1. It was measured that the conversion rate of vinylidene chloride was 98.9%, and the mass fraction of HFC-143a (without hydrogen chloride) in the outlet gas was 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com