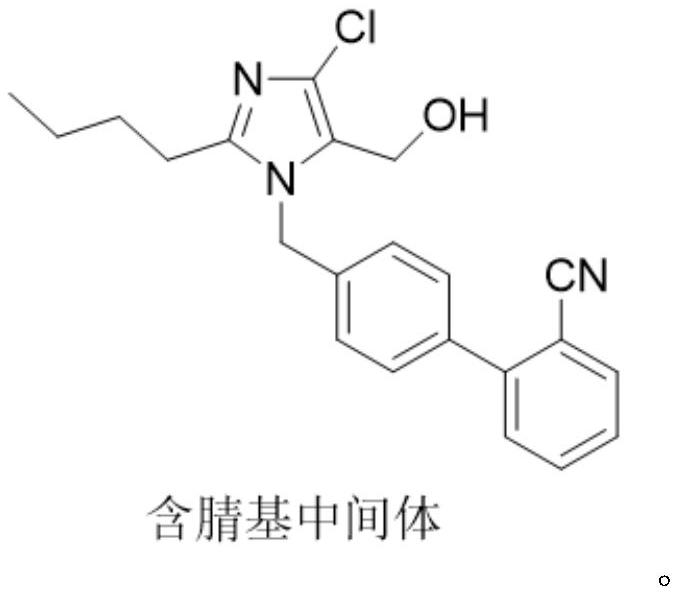
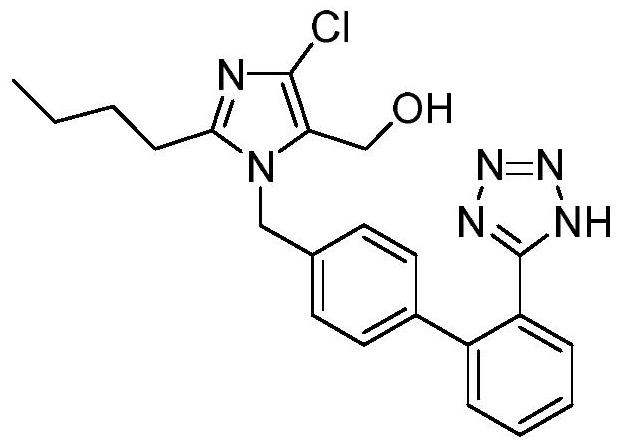
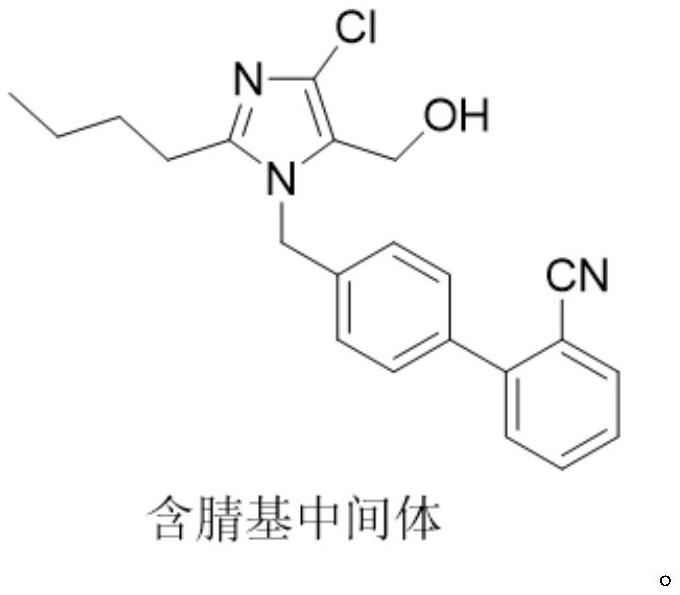
Preparation method of losartan
A technology of losartan and nitrile group, applied in the field of preparation of losartan, can solve the problems of inability to completely eliminate the risk of impurity contamination, high loss of losartan, entry of losartan and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] In the three-necked flask, add 30g of the nitrile intermediate, 9.8g of sodium azide, 12g of triethylamine hydrochloride, and 120ml of toluene, and control the temperature at 100-103°C to react. After the reaction, add 300ml of 20% sodium bicarbonate aqueous solution , control the temperature at 75°C, stir and wash for 1 hour, remove the lower water layer after washing; add 300ml of 20% sodium bicarbonate aqueous solution, stir and wash at 75°C for 1 hour, separate the intermediate material layer after washing, and azide The ion residue is about 80ppm. Add 150ml of drinking water to the separated intermediate material layer, stir at room temperature, slowly add dilute hydrochloric acid dropwise, adjust the pH to 4-5, crystallize, filter with suction, and dry to obtain 29.9g of losartan, yield 89.6%, purity: 98.8 %, azide: not detected (detection limit: 1 ppm); NDMA: not detected (detection limit: 0.03 ppm); NDEA: not detected (detection limit: 0.02 ppm).
example 2
[0036] In the there-necked flask, add 30g of nitrile-based intermediate, 9.8g of sodium azide, 12g of triethylamine hydrochloride, and 120ml of toluene, and control the temperature to react at 100-103°C. After the reaction, add 300ml of 18% sodium carbonate aqueous solution to control The temperature was 75°C, stirred and washed for 1 hour. After the washing, the lower water layer was removed, and 300ml of 18% sodium carbonate aqueous solution was added for washing, and the washing was performed three times. After washing, the intermediate material layer was separated, and the residual azide ion was about 50ppm. Add 150ml of drinking water to the separated intermediate material layer, stir at room temperature, slowly add dilute hydrochloric acid dropwise, adjust the pH to 4-5, crystallize, filter with suction, and dry to obtain 29.6g of losartan, with a yield of 88.7%, and a purity of: 98.8%, azide: not detected (detection limit: 1 ppm); NDMA: not detected (detection limit: 0.0...
example 3
[0038] In the there-necked flask, add 30g of nitrile-based intermediate, 9.8g of sodium azide, 12g of triethylamine hydrochloride, and 120ml of toluene, and control the temperature to react at 100-103°C. After the reaction, add 500ml of 20% potassium carbonate aqueous solution to control The temperature was 75°C, stirred and washed for 1.5 hours. After washing, the lower water layer was removed, and 500ml of 20% potassium carbonate aqueous solution was added to wash for three times. After washing, the intermediate material layer was separated, and the residual azide ion was about 20ppm. Add 120ml of drinking water to the separated intermediate material layer, stir at room temperature, slowly add dilute hydrochloric acid dropwise, adjust the pH to 4-5, crystallize, filter with suction, and dry to obtain 29.5g of losartan, yield 88.4%, purity: 98.8 %, azide (detection limit: 1 ppm): not detected; NDMA: not detected (detection limit: 0.03 ppm); NDEA: not detected (detection limit:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com