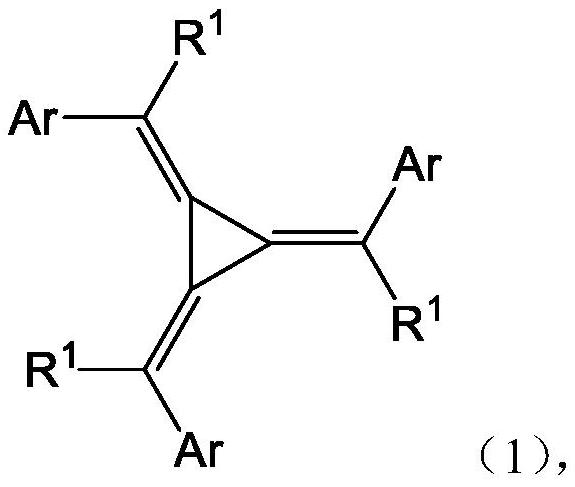
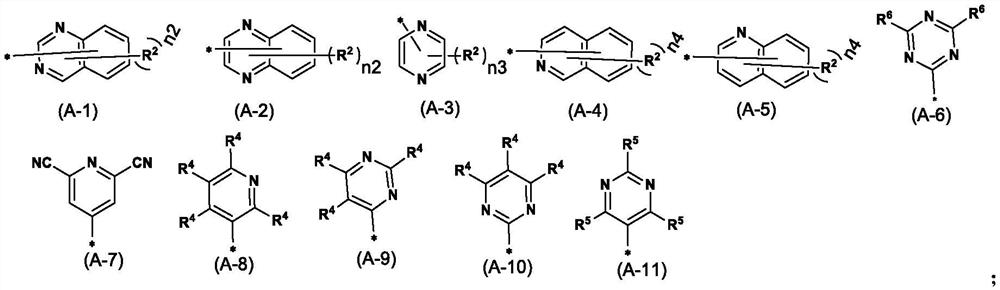
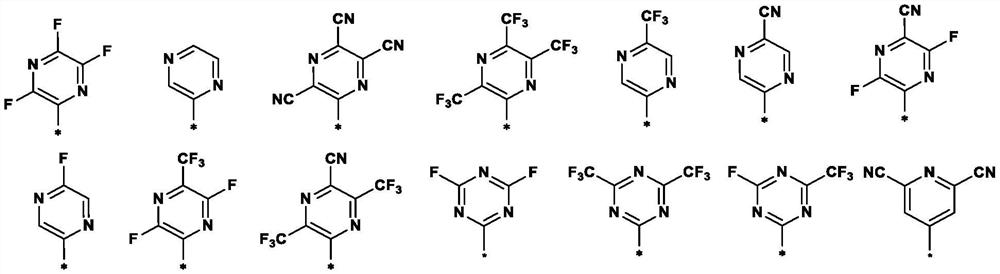
Nitrogen-containing heterocyclic ring substituted cyclopropane compound and application thereof
A technology of cyclopropane and nitrogen heterocycle, applied in the field of electroluminescent materials, can solve the problems of lifespan decline, insufficient stability of organic light-emitting diodes, and pollution of devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0141] The synthesis method of the compound according to the present invention is exemplified, but the present invention is not limited to the following examples.
[0142] 1. Compounds and synthetic steps
[0143] The target compound is as follows:
[0144]
[0145]
Embodiment 1
[0146] Embodiment 1: the synthesis of compound PD-1
[0147]
[0148] Synthesis of Compound PD-1
[0149] Under nitrogen protection, anhydrous tetrahydrofuran (230 mL) and lithium hydride (0.88 g) were added to the dry reaction kettle. Cool down to 10-15°C, dissolve compound 1 (4mmol) in anhydrous tetrahydrofuran (30mL), and slowly add it dropwise into the reaction kettle (keep the temperature below 15°C). After the dropwise addition, stir for 45min, then cool down to 0-5°C; Dissolve pentachlorocyclopropane (1mmol) in anhydrous tetrahydrofuran (11mL), slowly add dropwise to the reaction kettle, after the dropwise addition, naturally warm up to room temperature, and react for 44h. After the reaction was monitored by TLC and MS, water (30mL) was slowly added to the reaction kettle. After the reaction solution was acidified with concentrated hydrochloric acid, ethyl acetate (30mL) was added and stirred for 30min. Extract, combine the organic phases, wash with saturated brine...
Embodiment 2
[0151] Embodiment 2: the synthesis of compound PD-2
[0152]
[0153] Synthesis of compound PD-2
[0154] Under the protection of nitrogen, anhydrous tetrahydrofuran (230 mL) and lithium hydride (0.88 g) were added into the dry reaction kettle. Cool down to 10-15°C, dissolve compound 2 (4mmol) in anhydrous tetrahydrofuran (30mL), and slowly add it dropwise into the reaction kettle (keep the temperature below 15°C). After the dropwise addition, stir for 45min, then cool down to 0-5°C; Dissolve pentachlorocyclopropane (1mmol) in anhydrous tetrahydrofuran (11mL), slowly add dropwise to the reaction kettle, after the dropwise addition, naturally warm up to room temperature, and react for 44h. After the reaction was monitored by TLC and MS, water (30mL) was slowly added to the reaction kettle. After the reaction solution was acidified with concentrated hydrochloric acid, ethyl acetate (30mL) was added and stirred for 30min. Extract, combine the organic phases, wash with satura...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com