Fluorene ring group-containing non-condensed ring organic small molecular material and preparation method and application thereof
A small molecule and organic technology, applied in the field of organic photovoltaic materials, can solve the problems of unstable shape, limited sustainable development, weak light absorption, etc., and achieve the effect of improving solubility, reducing synthesis difficulty, and good film formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
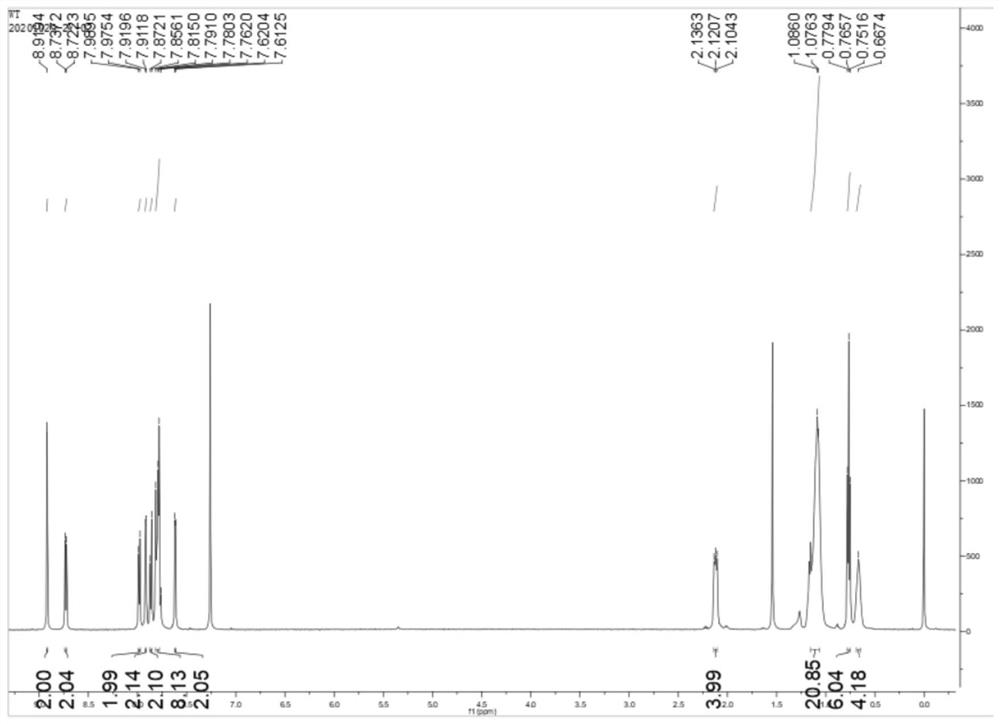
[0068] The structural formula of the non-condensed ring organic small molecule material Flu-H of this embodiment is as follows:
[0069] wherein R1 is H.
[0070] The preparation steps are as follows:
[0071] Step 1): the preparation of compound 1, the equation is as follows:
[0072]
[0073] Under nitrogen atmosphere, 2,7-bis(4,4,5,5-tetramethyl-1,3-dioxo-2-boryl)-9,9-dioctylfluorene (0.5g, 0.78mmol) and 5-bromothiophene-2-carbaldehyde (0.45g, 2.35mmol) were dissolved in 35mL phase transfer agent tetrahydrofuran, potassium carbonate aqueous solution (1M, 7mL) and tetrakistriphenylphosphine palladium (0.04g, 0.04mmol ) was added to the mixture, and reacted at 80°C for 16h. After the reaction was completed, add water to quench, add dichloromethane to extract the aqueous layer, dry over anhydrous sodium sulfate, remove the solvent by distillation under reduced pressure, and further purify by column chromatography. The eluent is petroleum ether and dichloromethane (3:1,...
Embodiment 2
[0078] The structural formula of the non-condensed ring organic small molecule material Flu-F of this embodiment is as follows:
[0079] where R1 is F.
[0080] The preparation steps are as follows:
[0081] Step 1): same as embodiment 1 step 1)
[0082] Step 2): The difference from step 2) of Example 1 is that 5,6-difluoro-3-(dicyanomethylene) indoketone (0.9g, 4.1mmol) is used to carry out knoevenagel reaction with compound 1, Finally, Flu-F (530 mg, 72%) was obtained as a black solid substance.
Embodiment 3
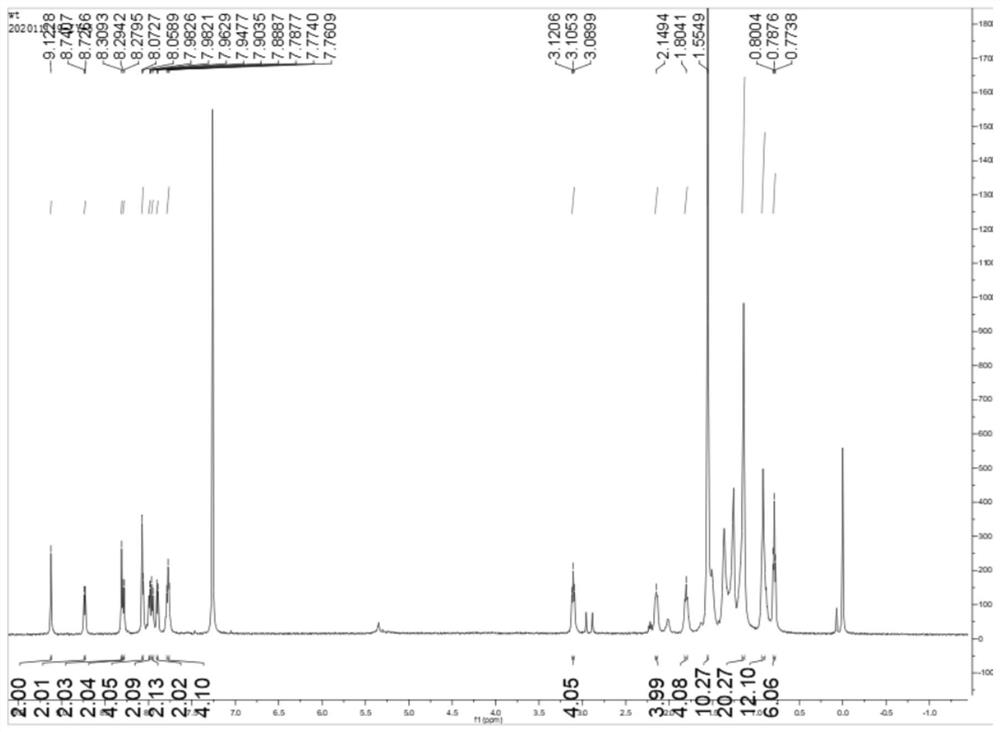
[0084] The structural formula of the non-condensed ring organic small molecule material BTT6IC-H of this embodiment is as follows:
[0085] wherein R2 is H.
[0086] The preparation steps are as follows:
[0087] Step 1): the preparation of compound 2, the equation is as follows:
[0088]
[0089] Under nitrogen atmosphere, 3-hexylthiophene (9.12g, 54mmol) was dissolved in 250mL of anhydrous tetrahydrofuran, and n-butyllithium (23.85mL, 2.5M in hexane, 59.5mmol) was added dropwise at -78°C. React at -78°C for 1 h, then add tributyltin chloride (19.37 g, 59.5 mmol), slowly rise to room temperature, and react at room temperature for 12 h. After the reaction, the reaction system was quenched by adding distilled water, and the aqueous layer was extracted with petroleum ether. The extracted organic layer was dried with anhydrous sodium sulfate, and the solvent was removed by distillation under reduced pressure to obtain compound 2 without further purification (24.1g, 97% )....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com