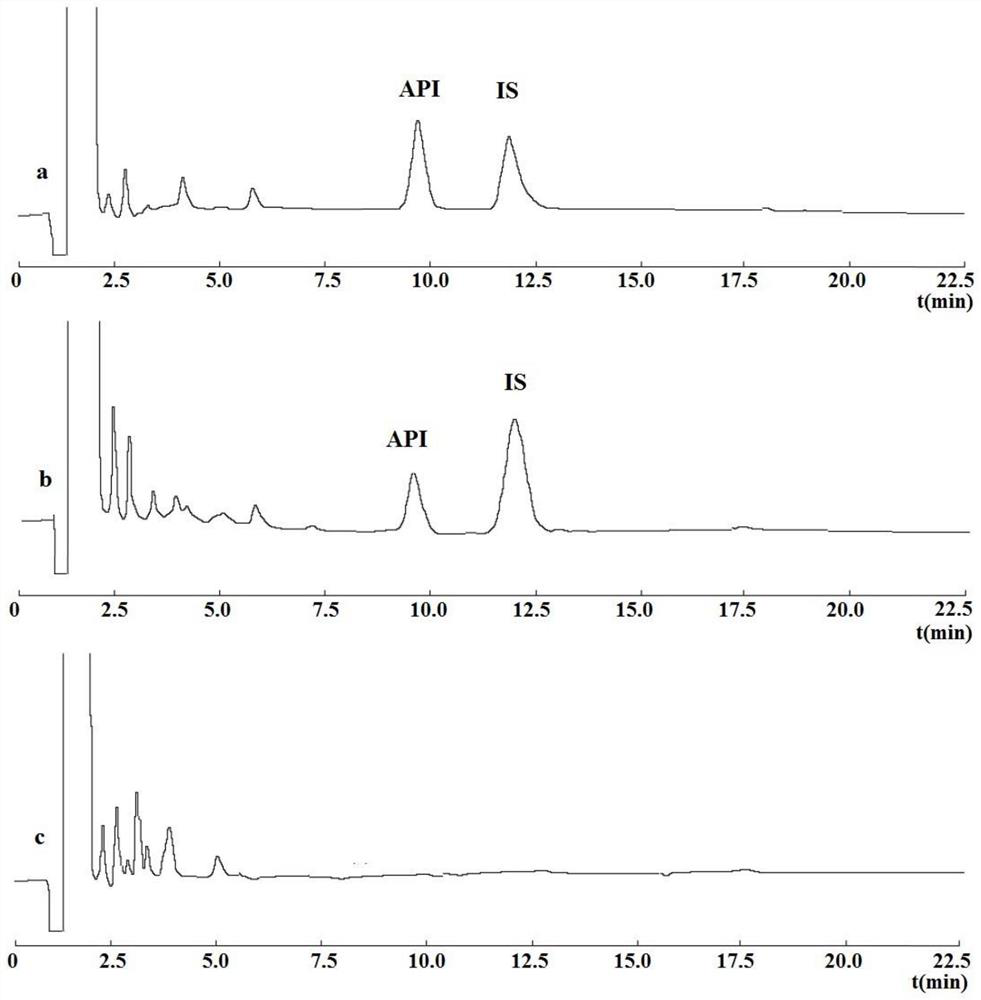
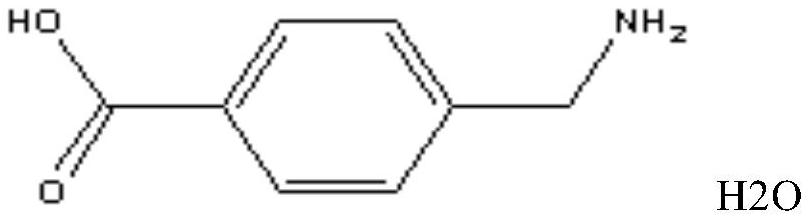
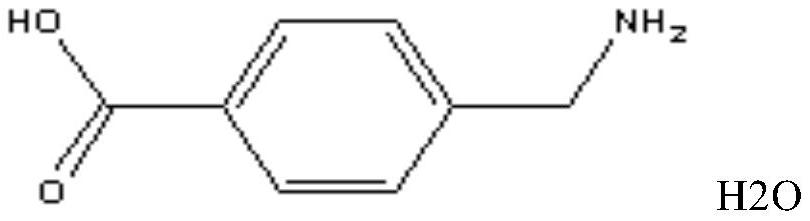
aminotoluic acid freeze-dried preparation
A technology of aminotoluic acid and freeze-dried powder injection, which is applied in the field of aminotoluic acid freeze-dried preparations, can solve problems such as inability to block, and achieve the effect of excellent methodological characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0148] Preparation Example 1. Preparation of powder injection containing aminotoluic acid
[0149] formula:
[0150] aminotoluic acid 100mg, Mannitol 95mg, Dextran-20 5mg, pH adjuster to pH 4.0, Water for Injection Appropriate amount, add to 4ml.
[0151] Preparation:
[0152] (1) Weigh the main drug and auxiliary materials of the recipe, place them in a stainless steel bucket, add about 80% of the water for injection in the recipe, dissolve each component, and then add 0.1% (w / v) of activated carbon by solution volume, Stir for 30 minutes, filter and decarbonize, and add water for injection to the full amount of the prescription.
[0153] (2) Sampling the filtrate, measure the pH value, and adjust it to a specified value with a pH regulator if necessary (the specified value is the pH value measured by diluting the dry powder obtained by freeze drying into a solution containing 10 mg / ml of active ingredient with water for injecti...
preparation example 2
[0157] Preparation Example 2. Preparation of powder injection containing aminotoluic acid
[0158] formula:
[0159]
[0160]
[0161] Preparation:
[0162] (1) Weigh the main drug and auxiliary materials of the recipe, place them in a stainless steel bucket, add about 80% of the water for injection in the recipe, dissolve each component, and then add 0.1% (w / v) of activated carbon by solution volume, Stir for 30 minutes, filter and decarbonize, and add water for injection to the full amount of the prescription.
[0163] (2) Sampling the filtrate, measure the pH value, and adjust it to a specified value with a pH regulator if necessary (the specified value is the pH value measured by diluting the dry powder obtained by freeze drying into a solution containing 10 mg / ml of active ingredient with water for injection, The same below), and then add water for injection to the full amount of the prescription.
[0164] (3) The medicinal liquid is filtered with a 0.45um micr...
preparation example 3
[0167] Preparation Example 3. Preparation of powder injection containing aminotoluic acid
[0168] formula:
[0169] aminotoluic acid 100mg, Mannitol 150mg, Dextran-40 2mg, pH adjuster to pH3.5, Water for Injection Appropriate amount, add to 5ml.
[0170] Preparation:
[0171] (1) Weigh the main drug and auxiliary materials of the recipe, place them in a stainless steel bucket, add about 80% of the water for injection in the recipe, dissolve each component, and then add 0.1% (w / v) of activated carbon by solution volume, Stir for 30 minutes, filter and decarbonize, and add water for injection to the full amount of the prescription.
[0172] (2) Sampling the filtrate, measure the pH value, and adjust it to a specified value with a pH regulator if necessary (the specified value is the pH value measured by diluting the dry powder obtained by freeze drying into a solution containing 10 mg / ml of active ingredient with water for injecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com