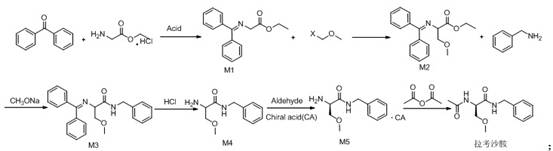
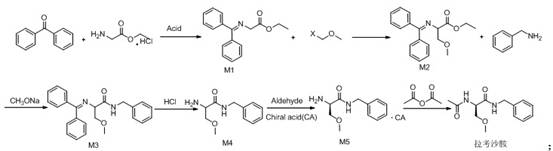
Synthetic route of lacosamide
A technology of lacosamide and synthesis route, applied in the field of medicinal chemistry, can solve the problems of low yield, complex route, large environmental pollution and the like, and achieves the effects of high yield, avoidance of use and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 50g of glycine ethyl ester hydrochloride in water, add sodium bicarbonate to adjust pH7-8, add dichloromethane for extraction, concentrate the dichloromethane layer under reduced pressure to obtain glycine ethyl ester oil; add toluene to glycine ethyl ester oil And 50g of benzophenone and catalytic amount of p-toluenesulfonic acid, reflux water separation; after the reaction is completed, add water to extract after cooling, concentrate the toluene layer, add petroleum ether to carry out crystallization, dry to obtain 61.88g of intermediate M1, yield 84.92 %, purity 99.3%, 1 H NMR (500MHz, CDCl 3 ), 7.41-7.7 (m, 10H, CH), 4.51 (s, 2H, NCH 2 ), 4.13(t,2H,OCH 2 ), 1.29(t,3H,CH 3 ).
Embodiment 2
[0029] Dissolve 50g of intermediate M1 and a catalytic amount of NaI in DMF, cool down and add sodium hydride, stir for 1h, add chloromethyl methyl ether dropwise, and raise the temperature to 55°C for reaction; after the reaction is complete, add dichloromethane and Extracted with water, concentrated dichloromethane, purified, dried to obtain 45.1g of intermediate M2, yield 78%, purity 99.5%, 1 HNMR (500MHz, CDCl 3 ), 7.41-7.7(m,10H,CH), 4.31(d,H,NCH), 4.13(t,4H,OCH 2 ), 3.3(s,3H,OCH 3 ), 1.29(t,3H,CH 3 ).
Embodiment 3
[0031] Dissolve 30g of intermediate M2 and 11.5g of benzylamine in ethanol, add 0.1 times of newly prepared sodium ethoxide (sodium block dissolved in ethanol) to reflux reaction; after the reaction is completed, concentrate ethanol under reduced pressure, add dichloromethane and water for extraction, and concentrate di Chloromethane layer, purified, dried to obtain 28.2g intermediate M3, yield 79%, purity 99.05%, 1 HNMR (500MHz, CDCl 3 ), 7.9(m,2H,CH), 7.58-7.62(m,8H,CH), 7.23-7.33(m,5H,CH), 4.3-4.4(s,2H,NCH 2 ), 4.45 (m, H, NCH), 3.85-4.18 (d, 2H, OCH 2 ), 3.3(s,3H,OCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com