Pyridone hexa-alkyne amine modified derivative and preparation method and application thereof
A technology of pyridone and its derivatives, which is applied in the field of pyridone modified derivatives of six-alkyne amine and its preparation and application, and can solve the problems that cannot be effectively and completely cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
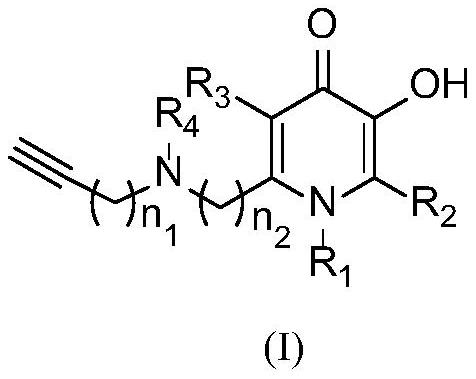
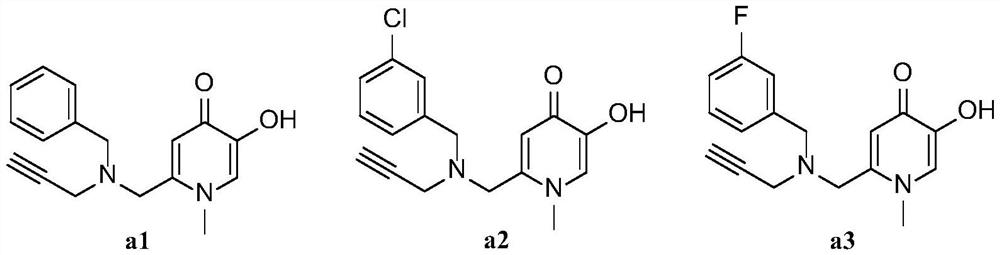
[0086] The preparation method of 1-methyl-2-((benzyl(propargyl)amino)methyl)-5-hydroxypyridin-4(1H)-one (a1)
[0087] Kojic acid (12.78g, 90mmol), benzyl bromide (23.08g, 135mmol), and potassium carbonate (24.84g, 180mmol) were added to a 500mL single-necked bottle, and reacted at 60°C for 5h under the condition of ethanol as the solvent (250mL). After the reaction, the reaction system was concentrated under reduced pressure to obtain the crude product as a light yellow solid, which was washed with water and dried to obtain a white solid (18.75 g), with a yield of 89.8%.
[0088] Take the above white solid (13.92g, 60mmol), dissolve it in ethanol (150mL), then add sodium hydroxide (4.80g, 120mmol), 9.30g methylamine aqueous solution (mass fraction: 40%; methylamine 120mmol), at 50 The reaction was carried out at ℃ for 2 hours. After the reaction, the reaction solution was concentrated under reduced pressure and purified by silica gel column chromatography (dichloromethane:meth...
Embodiment 2
[0095]The preparation method of 1-methyl-2-((3-chlorobenzyl (propargyl) amino) methyl)-5-hydroxypyridin-4(1H)-one (a2)
[0096] 3-Chlorobenzylamine (0.28g, 2mmol), potassium carbonate (0.55g, 4mmol) and N,N-dimethylformamide (10mL) were added to a 50mL single-necked bottle, and then 3 -Bromopropyne (0.12g, 1mmol) in N,N-dimethylformamide solution (2mL) was slowly added dropwise to the reaction solution, stirred at 30°C for 12h, after the reaction was completed, ethyl acetate (20mL) was added , extracted with 20 mL×3 saturated brine, combined the organic layers, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography (eluted with n-hexane:ethyl acetate=5:1), collected the The eluate of the compound (obtained by TLC spotting) was concentrated under reduced pressure to obtain a bright yellow transparent liquid (0.14 g), with a yield of 77.8%.
[0097] Take the above-mentioned bright yellow transparent liquid (0.1...
Embodiment 3
[0101] The preparation method of 1-methyl-2-((3-fluorobenzyl(propargyl)amino)methyl)-5-hydroxypyridin-4(1H)-one (a3)
[0102] 3-Fluorobenzylamine (0.25g, 2mmol), potassium carbonate (0.34g, 2.5mmol) and N,N-dimethylformamide (9mL) were added to a 50mL single-necked bottle respectively, and the 3-Bromopropyne (0.12g, 1mmol) in N,N-dimethylformamide solution (3mL) was slowly added dropwise to the reaction solution, stirred at 30°C for 12h, after the reaction was completed, ethyl acetate (15mL ), extracted with 20mL×3 saturated brine, combined the organic layers, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography (n-hexane: ethyl acetate = 5:1 elution), the collection containing The eluate of the target compound (obtained by TLC spotting) was concentrated under reduced pressure to obtain a bright yellow transparent liquid (0.11 g), with a yield of 67.5%.
[0103] Take the above-mentioned bright yellow transp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com