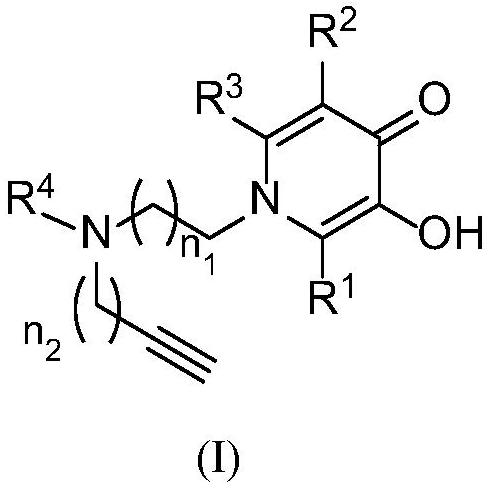
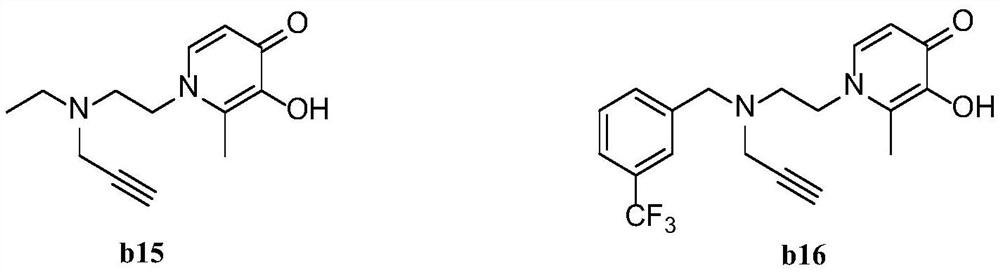
Pyridone-site alkyne amine modified derivative as well as preparation method and application thereof
A technology of pyridone and its derivatives, which can be applied in drug combination, organic chemistry, nervous system diseases, etc., and can solve problems such as oxidative stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
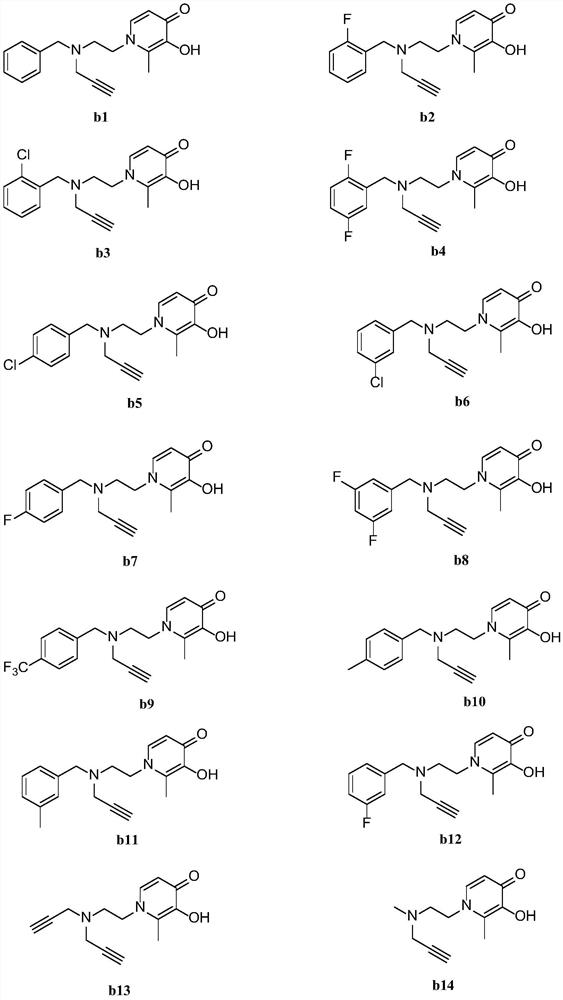
[0071] The preparation method of 1-(2-(benzyl(propargyl)amino)ethyl)-2-methyl-3-hydroxypyridin-4(1H)-one (b1)
[0072] Add maltol (41.58g, 330mmol), benzyl bromide (67.72g, 396mmol), anhydrous potassium carbonate (68.31g, 495mmol), acetone 600mL into a 2000mL single-necked bottle, react at 60°C for 4h, stop the reaction, and concentrate under reduced pressure The reaction solution was dissolved in 600mL of water, extracted with 600mL×4 dichloromethane, the organic layers were combined, washed 3 times with saturated brine, finally dried with anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid 2-methyl-3- Benzylpyran-4-one (68.77g), yield 96.5%.
[0073] Pyrone (64.80g, 300mmol), ethylenediamine (21.60g, 360mmol), sodium hydroxide (14.40g, 360mmol), ethanol (400mL), water (300mL), and React at 75°C for 1.5h. After the reaction, concentrate the reaction solution under reduced pressure, purify it by silica gel column chromatography (dichloromethane: methanol: am...
Embodiment 2
[0081] The preparation method of 1-(2-(2-fluorobenzyl(propargyl)amino)ethyl)-2-methyl-3-hydroxypyridin-4(1H)-one (b2)
[0082] Get the formula 3 intermediate (0.52g, 2mmol) obtained from the reaction of Example 1, anhydrous potassium carbonate (0.41g, 3mmol) is dissolved in DMF (4mL), get 3-bromopropyne (0.21g, 1.8mmol) and use DMF (2mL) was diluted in a constant pressure dropping funnel, added dropwise at 40°C for 6 hours, then concentrated the reaction solution under reduced pressure, and purified by silica gel column chromatography (dichloromethane:methanol:ammonia=20:1:0.1 for washing deagent), concentrated under reduced pressure to obtain formula 5 (0.37g), yield 69.4%
[0083] Add the intermediate of formula 5 (0.30g, 1mmol), potassium hydroxide (0.11g, 2mmol), 2-fluorobenzyl bromide (0.28g, 1.5mmol), tetrahydrofuran (15mL) into a 50mL single-necked bottle, and reflux at 69°C Reaction 20h. After the reaction, the reaction solution was concentrated, purified by silica g...
Embodiment 3
[0088] The preparation method of 1-(2-(2-chlorobenzyl (propargyl) amino) ethyl) -2-methyl-3-hydroxypyridin-4 (1H)-one (b3)
[0089] Get the formula 3 intermediate (1.29g, 5mmol) obtained by the above-mentioned embodiment 1 reaction, anhydrous potassium carbonate (1.03 g, 7.5mmol) is dissolved in DMF (10mL), take 3-bromopropyne (0.53g, 4.5mmol ) and diluted with DMF (7.5mL) in a constant pressure dropping funnel, added dropwise at 40°C for 6h, then concentrated the reaction solution under reduced pressure, purified by silica gel column chromatography (dichloromethane:methanol:ammonia=20:1: 0.1 as eluent), concentrated under reduced pressure to obtain formula 5 (0.96g), yield 72.1%.
[0090] Add the intermediate of formula 5 (0.30g, 1mmol), potassium hydroxide (0.11g, 2 mmol), 2-chlorobenzyl bromide (0.31g, 1.5mmol), tetrahydrofuran (15mL) into a 50mL single-necked bottle, and at 69°C After 20 hours of reflux reaction, the reaction solution was concentrated, purified by silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com