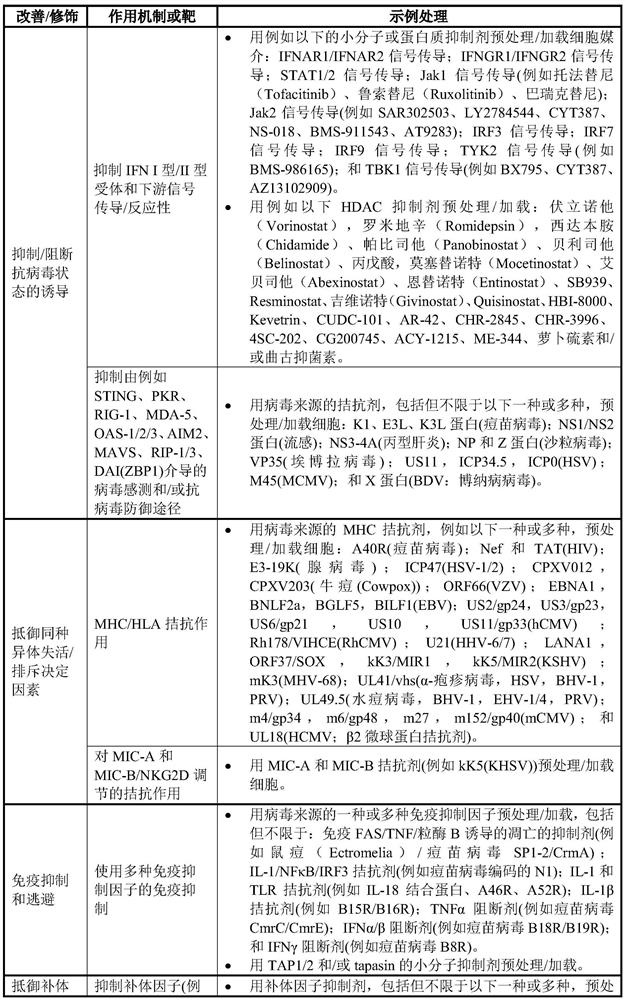
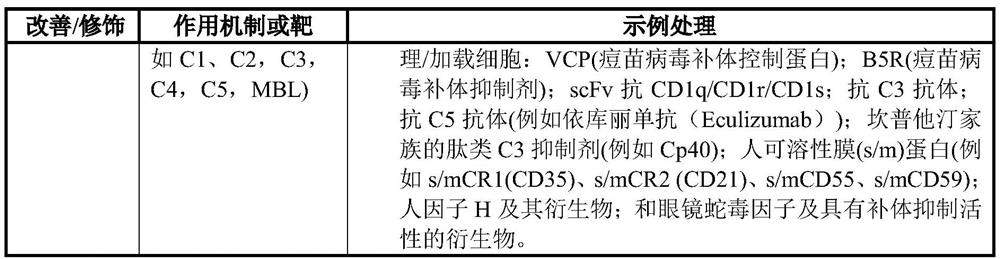
Cell-based vehicles for potentiation of viral therapy
A technology of leukemia cells and cells, applied in the field of cell-based media for intensified virotherapy, which can solve the problems of oncolytic virus efficacy barriers and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0947] Cell Isolation and Culture
[0948] A. Adipose-derived stem cells (ADSC)
[0949] I. Extraction and preparation of adipose stromal vascular fraction
[0950] Administer local anesthesia to the subject with 0.5% lidocaine 0.5% and 1:400,000 epinephrine and 8.4% HCO 3 , titrated to pH 7.4 (typically 5cc HCO in 60cc total volume 3 ). Objects then utilize trademarks from CellSurgical Network, Beverly Hills, CA, USA (CSN) Liposuction is performed under the cell harvesting and closed system collection and processing system available under the device, which includes a fat handling unit (gas-tight syringe for liposuction) and a 2.5mm-3mm cannula. Bacitracin ointment and a Band-Aid are secured to the wound along with a compression bandage.
[0951] Expanded adipose stem cells derived from supra adventitial adipose stromal cells were used in the examples provided herein. Stromal vascular fractions (SVF) containing ADSCs were prepared in a closed system according to the fol...
Embodiment 2
[0983] Effect of Adipose-Derived Stem Cells (ADSCs) on Viral Amplification and Infection in the Presence or Absence of Subject-derived Immune Cells (Peripheral Blood Mononuclear Cells (PBMCs))
[0984] For a particular virus, a vector cell that amplifies the virus is identified as a candidate for delivering the virus to a subject for viral therapy. This example evaluates the effect of subject-derived immune cells on the ability of vector cells to promote viral amplification and infection. PBMCs are used for this purpose. Factors affecting viral infection and amplification of vector cells include, for example, the presence or absence of interferon (IFN) and / or the presence or absence of PBMCs, as assessed by appropriate assays that measure the amount of virus present. Such assays include, for example, viral plaque assays (VPA), qPCR (or any other assay that measures the genomic amount of viral DNA present), ELISA (assays for virally encoded or engineered reporter proteins or e...
Embodiment 3
[1009] Virus amplification and infection in vector cell + tumor cell co-cultures
[1010] Amplification and infection of oncolytic viruses in the presence of different tumor / cancer cell types was evaluated to determine whether specific vector cell and oncolytic virus combinations are effective for tumor / cancer cell oncolysis. In this way, tumors can be identified as resistant or permissive to viral infection, and any enhancement of viral delivery from vector cells to tumor cells can be assessed. Other factors such as the effect of IFNγ pretreatment, carrier cell dosage, and secretion of soluble factors by carrier cells can also be assessed. This example evaluates the effect of an exemplary carrier cell type ADSC on the ability to deliver an exemplary virus (vaccinia virus) to an exemplary cancer cell line (B16 melanoma cells).
[1011] A. ADSCs promote oncolysis of resistant and permissive tumor cell lines
[1012] For example, fluorescence microscopy, plaque assay analysis,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com