Application of myeloid cell triggering receptor 2 as a novel coronavirus pneumonia diagnosis or treatment target
A coronavirus and cell technology, applied in disease diagnosis, antiviral agents, respiratory diseases, etc., can solve the problems of high disease mortality rate and poor effect of severe patients, and achieve less trauma, less pain, and easy review Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
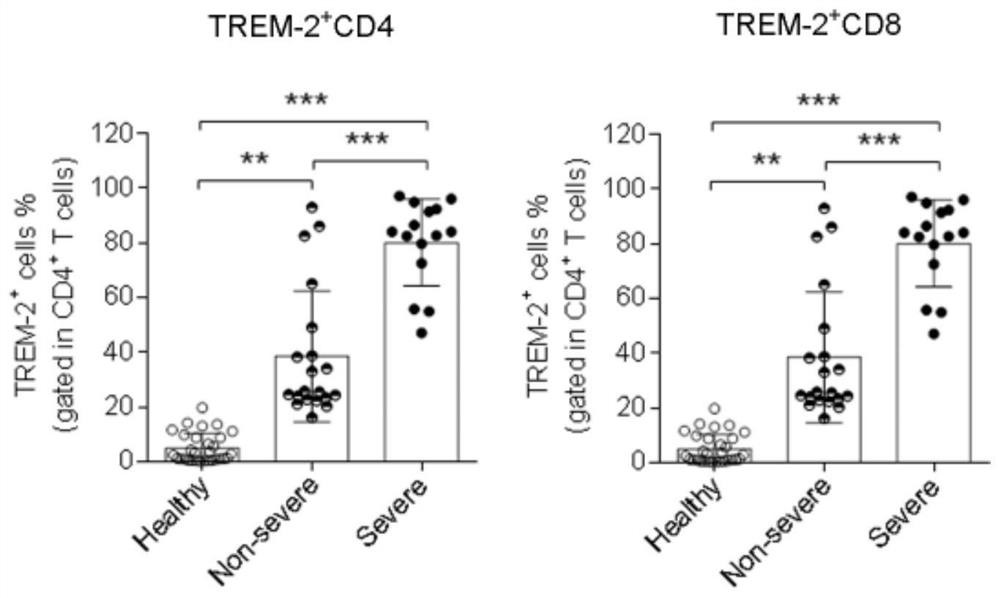
[0040] Select 15 cases of severe (Severe) and 20 cases of non-severe (Non-severe) patients with novel coronavirus pneumonia as the experimental group; select 20 cases of healthy people as the control group.
[0041] Healthy subjects: subjects without clinical symptoms.
[0042] Patients with novel coronavirus pneumonia: SARS-CoV-2 virus nucleic acid test positive, and clinical symptoms manifested as pneumonia.
[0043] Collect 5 mL of peripheral blood from subjects in the experimental group and control group, lyse red blood cells to prepare a single-cell suspension, and adjust the total amount of cells to 5×10 6 For each, add TREM-2 antibody (R&D Company, Cat. No. FAB1278P) and CD4 and CD8 antibodies (both purchased from Biolegend Company) to 100 μL of the system, mix well and protect from light for 30 minutes, and detect by flow cytometry, with FCS / SSC as the gate Analyze the proportion of TREM-2 positive cells in CD4 and CD8 positive cells respectively.
[0044] figure 1 ...
Embodiment 2
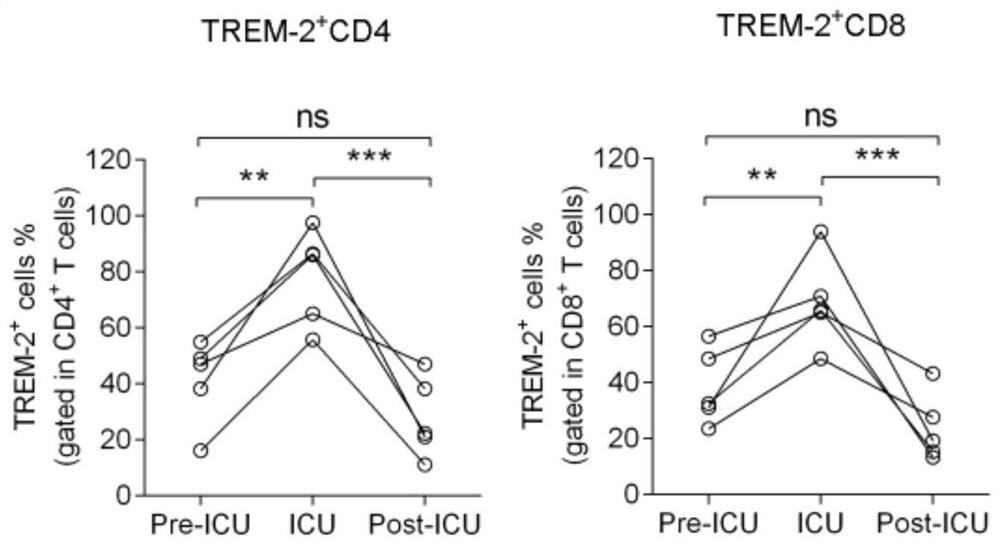
[0046] Five cases of critically ill patients with novel coronavirus pneumonia were selected, and the expression of TREM-2 in patients in the early stage (Pre-ICU), severe stage (ICU) and remission stage (Post-ICU) were detected respectively.
[0047] Refer to the "New Coronary Pneumonia Diagnosis and Treatment Program (Trial Seventh Edition)" for the judgment of the above-mentioned severe stage, and take 5 mL of peripheral blood from the patient in 3 periods for the following tests. The red blood cells of the above samples were lysed to prepare a single cell suspension, and the total amount of cells was adjusted to 5×10 6 For each, add TREM-2 antibody (R&D Company, Cat. No. FAB1278P) to 100 μL of the system, mix well and protect from light for 30 minutes, and perform flow cytometry detection. FCS / SSC gates are set to analyze the percentage of TREM-2 positive cells that are CD4 and CD8 positive, respectively. cell ratio.
[0048] figure 2 It is the expression level of TREM-2...
Embodiment 3
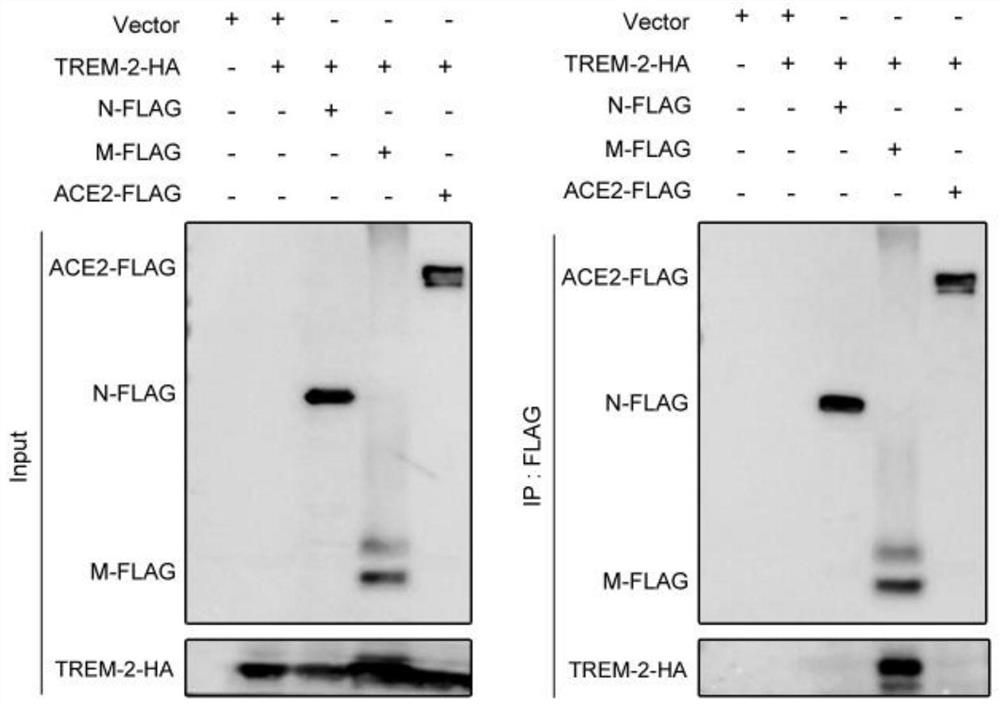
[0050] The result is as image 3 As shown, a total of 5 kinds of plasmids were constructed, including Vector blank plasmid, TREM-2 expression plasmid with HA tag (TREM-2-HA), SARS-CoV-2 membrane protein expression plasmid with FLAG tag (M-FLAG), SARS-CoV-2 nucleoprotein expression plasmid with FLAG tag (N-FLAG), angiotensin converting enzyme 2 (ACE2) expression plasmid with FLAG tag (ACE2-FLAG).
[0051] The above 5 kinds of plasmids were combined into 293T cells as shown in the figure, - indicates that the plasmid is not transferred, + indicates that the plasmid is transferred. After 24 hours of transfer, the cells were collected, lysed to obtain proteins, and co-immunoprecipitated to detect the combination of TREM-2 and viral proteins. Input on the left indicates the expression of the above plasmids in the cell lysate detected by immunoblotting. The results show that the above plasmids can be effectively expressed in 293T cells. The black bands are ACE2-FLAG and N-FLAG from...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com