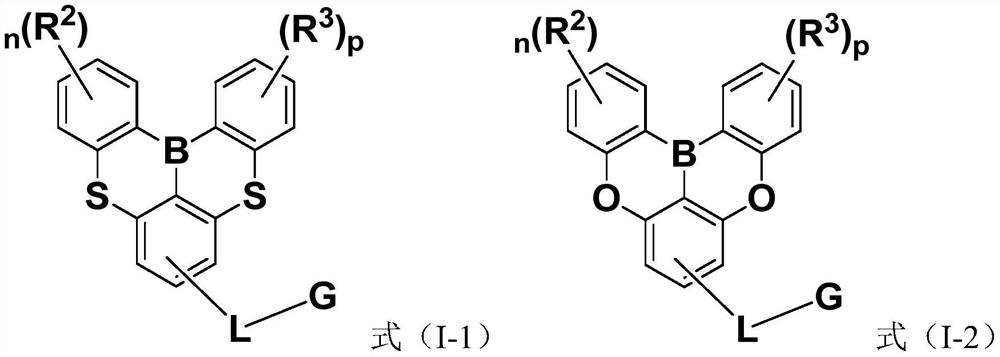
Compounds and application thereof
A compound, unsubstituted technology, applied in the field of organic electroluminescence, can solve the problems of OLED product efficiency, life, cost, etc. that cannot be completely solved, and achieve the effect of improving charge transport performance, improving luminous efficiency, and reducing voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0130] Synthesis of Compound P2
[0131]
[0132] Under nitrogen atmosphere, M2 (23.03g, 50mmol), 1-bromo-3,5-difluorobenzene (9.65g, 50mmol), tridibenzylideneacetone dipalladium (Pd 2 (dba) 3 , 0.91g, 1mmol), 2-dicyclohexylphosphine-2',6'-dimethoxybiphenyl (s-phos, 0.82g, 2mmol), t-BuONa (9.60g, 100mmol), toluene 250mL Put it into a 1L reaction vessel, and reflux at 110°C for 12h. Cool to room temperature, combine and concentrate the organic phases. Separation by column chromatography yielded 23.13 g of intermediate P2-1. Molecular weight calculated: 572.66, found C / Z: 572.7.
[0133]
[0134] Under nitrogen atmosphere, put P2-1 (22.91g, 40mmol), phenol (9.41g, 100mmol), cesium carbonate (32.3g, 100mmol), N,N-dimethylformamide 200mL into a 1L reaction vessel, Reflux reaction at 150°C for 12h. Cool to room temperature, combine and concentrate the organic phases. Separation by column chromatography yielded 23.14 g of intermediate P2-2. Molecular weight calculated:...
Synthetic example 2
[0138] Synthesis of Compound P7
[0139] Replace M2 in Synthesis Example 1 with M1 of the same amount, and keep the other things unchanged, to obtain P7. Molecular weight calculated: 726.64, found C / Z: 726.6.
Synthetic example 3
[0141] Synthesis of Compound P11
[0142] Replace M2 in Synthesis Example 1 with 7H-benzo[C]carbazole in the same amount, and keep the others unchanged, to obtain P11. Molecular weight calculated: 485.35, found C / Z: 485.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com