Preparation method of 2, 6-dioxabicyclo-(3.3.0)-octane-3,7-dione
A technology for dioxa and octane, which is applied in the field of preparation of 2,6-dioxabicyclo-octane-3,7-dione, can solve the problems of high price, unfavorable industrialized production, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
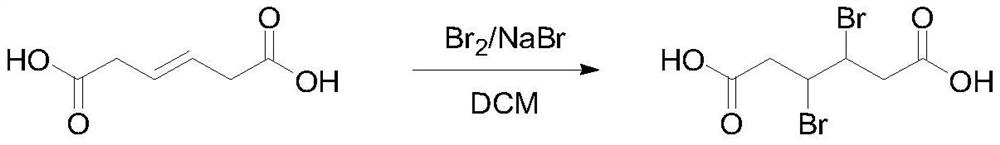
[0024] Add 144g (1.0mol) hexa-3-enedioic acid, 2.1g sodium bromide and 500mL dichloromethane into the reaction flask, cool the reaction solution to 0°C and slowly add 168g (1.05mol) bromine and 200mL dichloromethane Mixed solution of methane, the dropwise addition process controlled the temperature at 0-10°C, after the dropwise addition was completed, the reaction was stirred overnight at room temperature. TLC detected that the reaction of raw materials was complete, quenched with saturated sodium bisulfite aqueous solution, washed with saturated brine, distilled the solvent under reduced pressure, filtered the precipitated solid, and rinsed with a small amount of ice water and n-heptane to obtain 257.1 g of 3,4-dibromoadipic acid. Yield 84.6%, HPLC 98.9%. 1 H NMR (400MHz, CDCl 3 ):11.23(s,2H),4.21-4.17(m,2H),2.71-2.66(m,4H).
Embodiment 2
[0026]
[0027] Add 144g (1.0mol) hexa-3-enedioic acid, 1.6g potassium bromide and 500mL chloroform into the reaction flask, cool the reaction solution to 0°C and slowly add 168g (1.05mol) bromine and 200mL chloroform mixed solution, During the dropwise addition, the temperature was controlled at 0-10°C. After the dropwise addition was completed, the reaction was stirred overnight at room temperature. TLC detected that the reaction of raw materials was complete, quenched with saturated sodium bisulfite aqueous solution, washed with saturated brine, distilled under reduced pressure to distill chloroform, solid precipitated, filtered, rinsed with a small amount of ice water and n-heptane to obtain 3,4-dibromoadipic acid 283.0 g, yield 93.1%, HPLC: 99.5%.
Embodiment 3
[0029]
[0030] Under nitrogen protection, 30.4g (0.1mol) 3,4-dibromoadipic acid and 210mL sulfolane were added to the reaction flask, and the material was cooled to 0°C, and 8.8g (0.22mol) 60% sodium hydride and 40mL tetrahydrofuran were slowly added After the dropwise addition is completed, stir and react at room temperature for 1 hour, add 4.4g of 15-crown-5, and then heat up to 90-95°C for reaction. During the reaction, the tetrahydrofuran solvent is distilled out under normal pressure, and then the heat preservation reaction is continued. After 6 hours, TLC detected that the reaction of the raw materials was complete. Then the temperature of the reaction solution was lowered, and distillation under reduced pressure (11 mmHg) was started, and the fraction at 77-83°C was collected to obtain 12.4 g of 2,6-dioxabicyclo-(3.3.0)-octane-3,7-dione. 87.6%, GC: 99.5%. 1 H NMR (400MHz, CDCl 3 ):5.04-4.98(m,2H),2.56-2.51(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com