Method for improving water solubility and bioavailability of hesperidin
A hesperidin and water-soluble technology, which is applied in the field of improving the water-solubility and bioavailability of hesperidin, can solve the problems of unsuitable preparation process, difficulty in cyclodextrin embedding, and inability to remove the solvent, so as to overcome the inability to remove the solvent , Improve oral bioavailability and avoid the use of toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
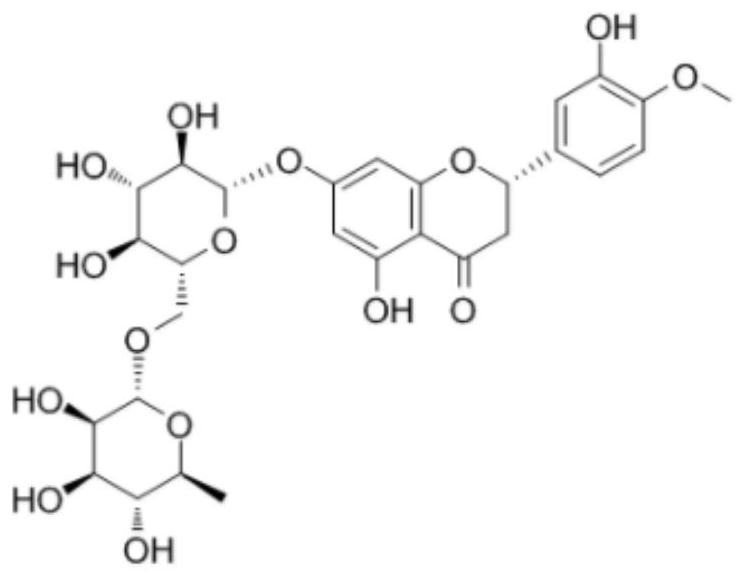
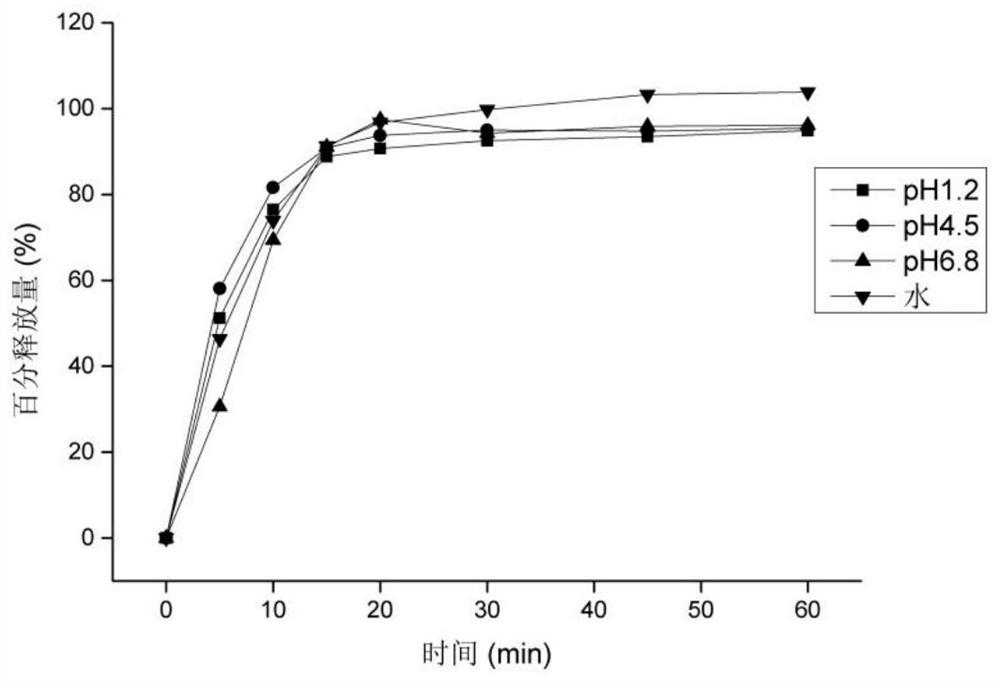
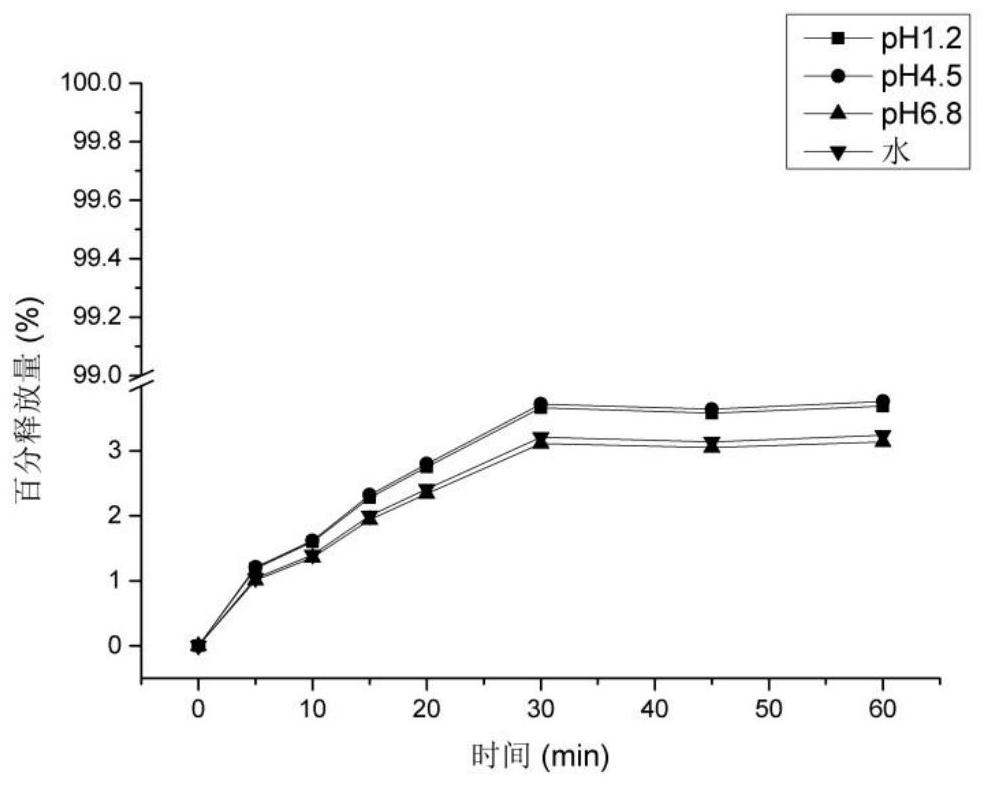
[0043] Weigh 10g of β-cyclodextrin, add 100mL of purified water, heat and stir at 50°C to completely dissolve the cyclodextrin, adjust the pH to 11.5 with triethylamine, add 5g of hesperidin raw material (such as figure 1 shown), maintain 50°C and continue stirring to completely dissolve the hesperidin. Stirring was continued at 50°C for 3 hours for full embedding. After the embedding was completed, it was dried under reduced pressure to remove part of triethylamine, and then spray-dried to obtain a hesperidin-β-cyclodextrin sample. Finally, 14.74 g of the product was obtained with a yield of 98.27%. The solubility of hesperidin before and after embedding was measured, and its solubility in water, pH 1.2, pH 4.5, and pH 6.8 was measured. Determine its water partition coefficient. The results showed that the solubility of hesperidin in water increased from 0.0165 mg / mL before inclusion to 5.9533 mg / mL after inclusion by cyclodextrin.
[0044] Table 1 Example 1 Hesperidin bef...
Embodiment 2
[0047] Weigh 25g of HP-β-cyclodextrin, add 200mL of purified water, heat and stir at 60°C to completely dissolve the cyclodextrin, adjust the pH to 11.8 with diethylamine, add 10g of hesperidin raw material (such as figure 1shown), maintain 60°C and continue stirring to completely dissolve the hesperidin. And continue stirring at 60° C. for 2 hours to fully embed. After the embedding is completed, spray-dry to obtain a hesperidin-β-cyclodextrin sample. Finally, 34.12 g of the product was obtained with a yield of 97.49%. The solubility of hesperidin before and after embedding was measured, and its solubility in water, pH 1.2, pH 4.5, and pH 6.8 was measured (37°C, shaking for 24 hours). Determine its water partition coefficient. The results showed that the solubility of hesperidin in water increased from 0.0165mg / mL before inclusion to 6.3327mg / mL after inclusion by HP-β-cyclodextrin.
[0048] Table 2 Example 2 Hesperidin before and after embedding solubility and oil-water p...
Embodiment 3
[0051] Weigh 30g of α-cyclodextrin, add 500mL of purified water, heat and stir at 40°C to completely dissolve the cyclodextrin, adjust the pH to 11.96 with ethylenediamine, add 6g of hesperidin raw material (such as figure 1 shown), maintain 40°C and continue stirring to completely dissolve the hesperidin. And continue to stir at 40°C for 6 hours to fully embed. After the embedding is completed, part of the ethylenediamine is removed by drying under reduced pressure, and then the loose hesperidin-α-cyclodextrin inclusion complex is obtained by freeze-drying. Finally, 44.27 g of the product was obtained with a yield of 98.38%. The solubility of hesperidin before and after embedding was measured, and its solubility in water, pH 1.2, pH 4.5, and pH 6.8 was measured (37°C, shaking for 24 hours). Determine its water partition coefficient. The results showed that after α-cyclodextrin inclusion, the solubility of hesperidin in water increased from 0.0165 mg / mL before inclusion to 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com