Medicine composition containing antibody coupling medicine, freeze-drying agent, and preparation method and purpose of medicine composition and freeze-drying agent
A drug and drying technology, applied in the direction of drug combination, drug delivery, anti-tumor drugs, etc., can solve the problem of lack of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] 2.1 Preparation of F0002-ADC
[0108] It can be prepared by conventional methods in the art. For example, F0002-ADC can be prepared according to Method 1 or Method 2 disclosed in Chinese patent document CN201810078006.9. In an embodiment, it is prepared by Example 3 disclosed in Chinese patent document CN201810078006.9.
[0109] 2.2 Main instruments and reagents
[0110] Freeze dryer TELSTAR LyoBeta 6PL; high performance liquid phase analysis system Waters E2695; chromatographic column TOSOHTSKgel G3000SWXL (5μm, 7.8mm×300mm); capillary electrophoresis analysis system Beckman Coulter PA800plus; Calibration instrument MolecularDevices SpectraMax m2e. Citric acid was purchased from Hunan Huari Pharmaceutical Co., Ltd.; sucrose was purchased from Merck; arginine hydrochloride was purchased from Shanghai Concord Amino Acid Co., Ltd.; polysorbate 20 was purchased from J.T.Baker.
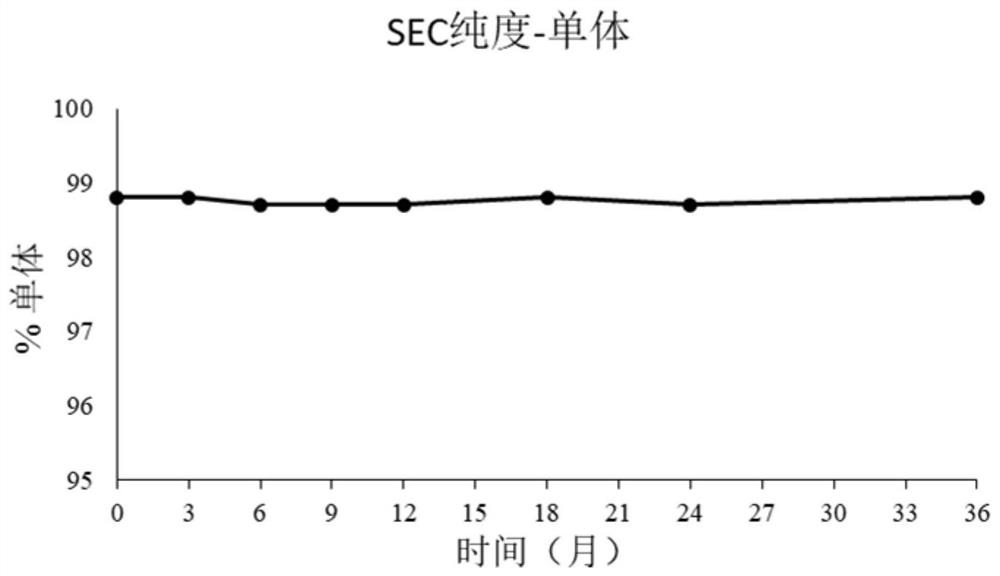
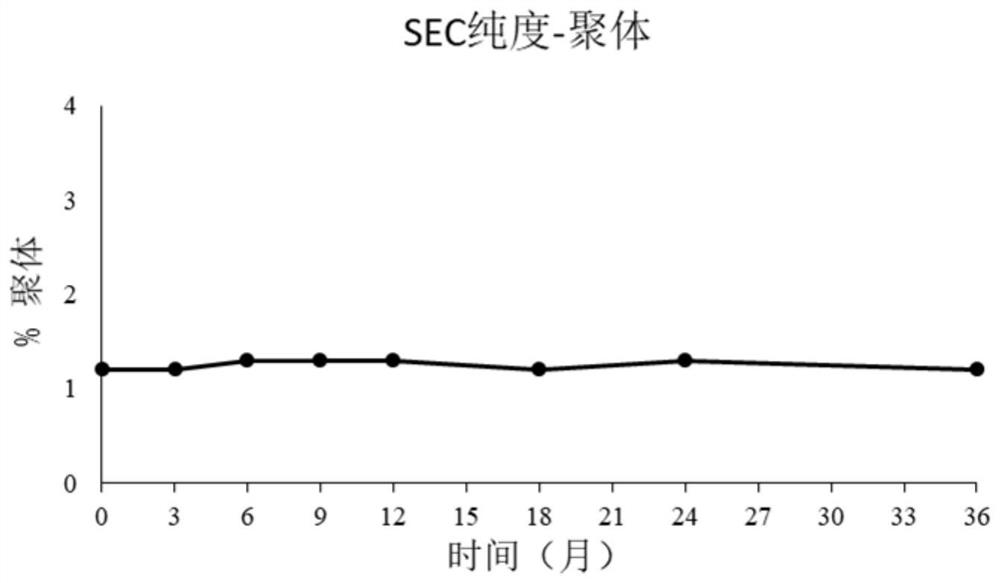
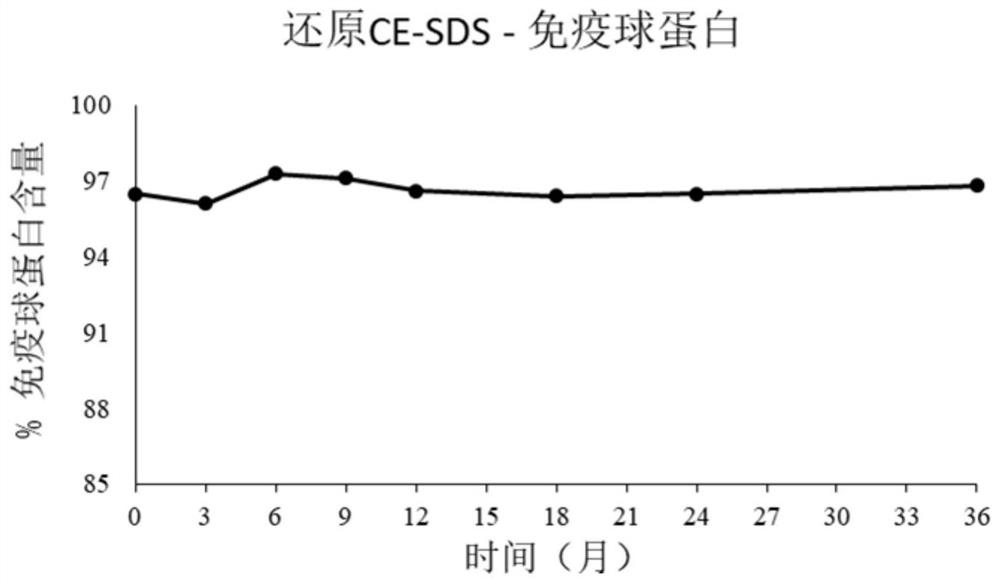
[0111] 2.3. Size Exclusion Chromatography (Size Exclusion Chromatography (SEC) Purity Analy...
Embodiment 1
[0122] The formulations and dissolution conditions of each pharmaceutical composition are shown in Table 1. In Table 1, in the formulation of each pharmaceutical composition, the concentration of citric acid-sodium citrate is 10 mM. Some pharmaceutical compositions can significantly improve the solubility of the F0002-ADC drug, and its drug solution is in a clear state, which is conducive to the development of stable preparations. Adding 20-50g / L arginine hydrochloride in the formula is the first choice to improve the solubility.
[0123] The preparation methods of the pharmaceutical composition all include the following steps (except for the final freeze-drying, F0002-ADC is in aqueous solution):
[0124] (1) Configuration of replacement buffer: Mix excipients other than antibodies, including citric acid-sodium citrate, sucrose, arginine hydrochloride, polysorbate 20 or polysorbate 80, and adjust the pH value to meet the table 1 as required;
[0125] (2) Use a 30kD ultrafi...
Embodiment 2
[0132] The water content of each pharmaceutical composition formulation and its freeze-dried preparation is shown in Table 2. In the formulations of each pharmaceutical composition in Table 2, the concentration of polysorbate 20 is 0.2 mg / mL, and the pH value is 5.0. The preparation of each pharmaceutical composition is as described in Example 1.
[0133] The preparation method of freeze-dried preparation is as follows:
[0134] The pharmaceutical composition containing F0002-ADC was divided into injection vials, and the injection vials containing the samples were placed in a LyoBeta 6PL lyophilizer to start lyophilization. Pre-freezing stage: Pre-cool for 1 hour below 5°C. Pre-cool for 1.5 hours below -5°C. Adjust the temperature of the board layer to below -45°C, and control the overall down-regulation rate below 1°C / min, and keep it for more than 2 hours. Slowly increase the temperature of the plate and anneal at no more than -25°C, and keep it for 2 hours. Rapidly coo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com