Preparation method of ramelteon
A kind of ramelteon, technology of compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
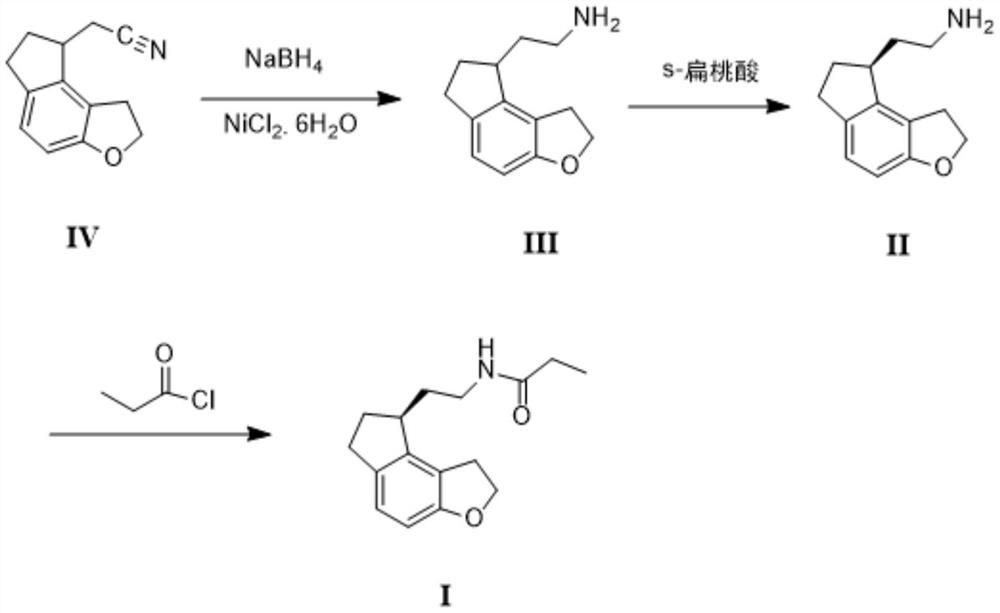
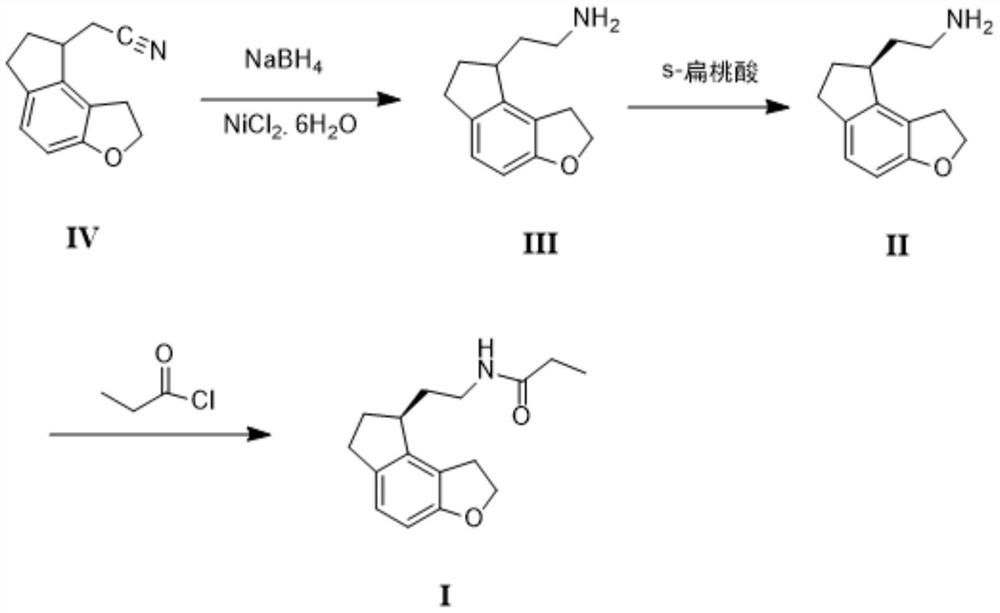
[0032] (1) In the reactor, add 19.7g (0.1mol) of compound IV, 270ml of ethanol, 30ml of water, 20g (0.084mol) of nickel dichloride hexahydrate, stir, and add 4.5g (0.12 mol) NaBH 4 After the addition, stir at 45°C for about 2-3 hours, cool, add 10ml of acetone and stir for 15 minutes, filter with suction, rinse the filter cake with 20ml of ethanol, spin the ethanol off after the filtrate is collected, add 30ml of water, and use ethyl acetate The ester was extracted 100ml×3 times, combined with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain 19.4g of compound III with a yield of 96.5%.
[0033] (2) Dissolve 19.4g (0.097mol) of compound III with 100ml of methyl tert-butyl ether, stir and heat up to 50°C, start to dropwise add a solution of 9g (0.06mol) of S-mandelic acid dissolved in 30ml of ethanol, 20 -30 minutes to complete the addition, the temperature was raised to 60...
Embodiment 2
[0036] (1) In the reactor, add 197g (1mol) of compound IV, 1500ml of ethanol, 300ml of water, 220g (0.93mol) of nickel dichloride hexahydrate, stir, and add 50g (1.32mol) of NaBH in batches within half an hour 4 After the addition, stir at 40°C for about 2 hours, cool, add 150ml of acetone and stir for 10 minutes, filter with suction, rinse the filter cake with 200ml of ethanol, collect the filtrate and spin off the ethanol under reduced pressure, add 300ml of water, and extract with ethyl acetate 400ml×3 times, combined with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain 192g of compound III with a yield of 95.5%.
[0037](2) Dissolve 192g (0.96mol) of compound III in 1000ml of methyl tert-butyl ether, stir and heat up to 50°C, start to drop a solution of 110g (0.73mol) of S-mandelic acid dissolved in 360ml of ethanol for 30 minutes After the addition, the temperature w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com