Anti-MMAE monoclonal antibody, coding sequence and application thereof
A monoclonal antibody and coding technology, applied in the field of biochemistry, can solve problems such as toxic and side effects, and achieve the effects of reducing toxic and side effects, good binding activity, and accelerating metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of anti-MMAE monoclonal antibody cDNA
[0023] (1) MMAE small-molecule antigen (obtained from Nanjing Lianning Biology) was mixed with complete Freund's adjuvant in equal volumes, and 4 4-5 week-old BALB / C female mice (obtained from the Southern Model Animal Center) were immunized. For the first immunization, the volume of each injection is 0.5ml;
[0024] (2) From the third week, mix 1 mg MMAE small molecule antigen with incomplete Freund's adjuvant in equal volume, immunize once every 3 weeks, the last immunization is 3-5 days before fusion, and immunize 5 times in total to stimulate B cells express antigen-specific antibodies;
[0025] (3) Antibody titer detection. For each mouse that has completed four immunizations, blood must be collected from the orbit, and then titer detection is performed. The detection time is generally 7-10 days after the fourth immunization.
[0026] (4) Myeloma cell preparation. One week before fusion, cryopreser...
Embodiment 2
[0033] Example 2: Cloning of Monoclonal Antibody Genes
[0034] (1) Acquisition of light and heavy chain genes: use mouse-specific primers to amplify the cDNA prepared in Example 1, clone the amplified DNA sequences of VH and VL into pUC19 vectors, and then carry out Transformation plated, picked clones for sequencing analysis.
[0035] (2) Analysis of the light and heavy chain variable region genes of the antibody: analyze the VH and VL according to the sequencing results, and finally obtain two light chain sequences and one heavy chain sequence (SEQ ID NO:5), combined with relevant biological information The final light chain sequence (SEQ ID NO: 6) was determined by chemical analysis, the nucleotide sequence of the heavy chain variable region was SEQ ID NO: 3, and the nucleotide sequence of the light chain variable region was SEQ ID NO: 4.
[0036] (3) Clone the light and heavy chain genes into the expression vector: clone the VH and VL sequences obtained from the analysi...
Embodiment 3
[0037] Example 3: Expression and purification of anti-MMAE antibody in eukaryotic expression system
[0038] (1) Cloning the correctly sequenced light and heavy chain genes into eukaryotic expression vectors pCAT1.0 and pCAT2.0, respectively;
[0039] (2) The cloned vector is further sequenced and identified, and then the correct clone is extracted into a plasmid;
[0040] (3) Culture HEK293F suspension cells, culture the cells in Freestyle 293 medium, when the density reaches 1×10 6 / ml, cell transfection was performed when the survival rate was >90%;
[0041] (4) Transfection method: the mass ratio of PEI / plasmid is 3:1, transfect according to the ratio of 1 μg plasmid / 1ml HEK293F cells;
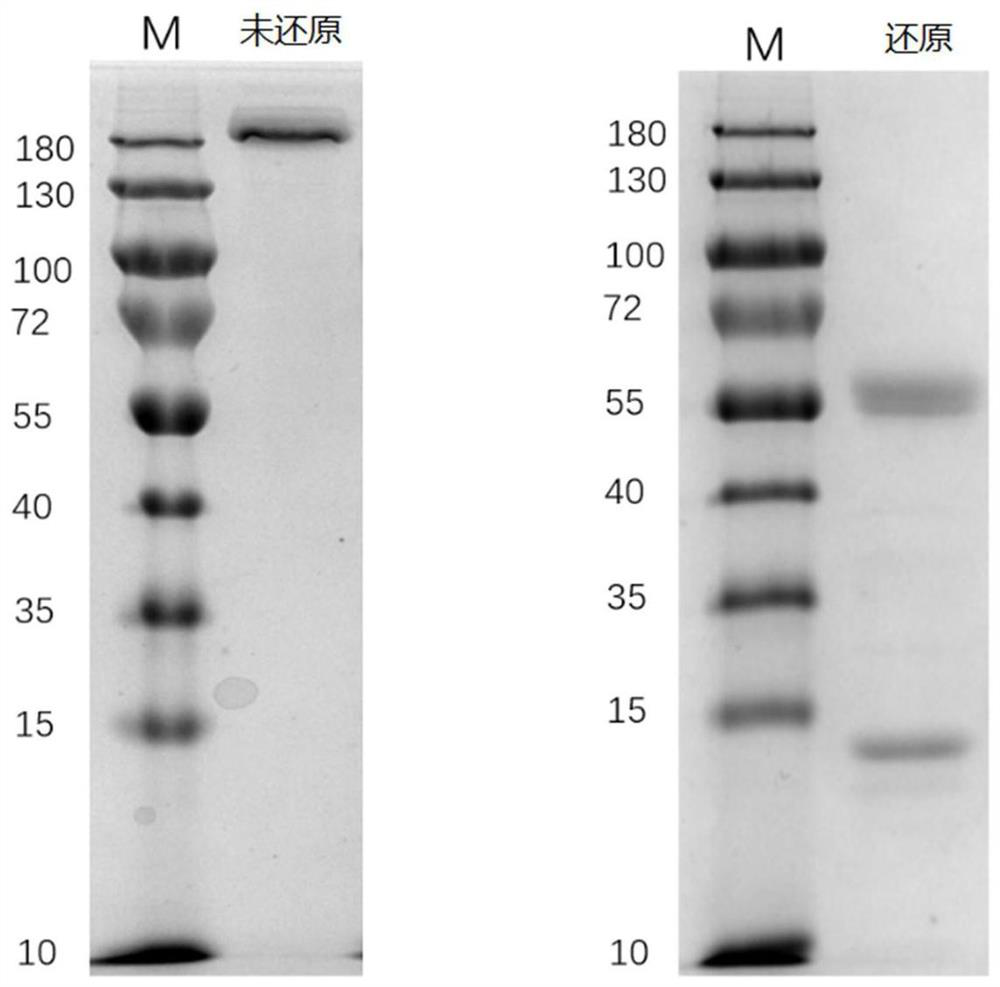
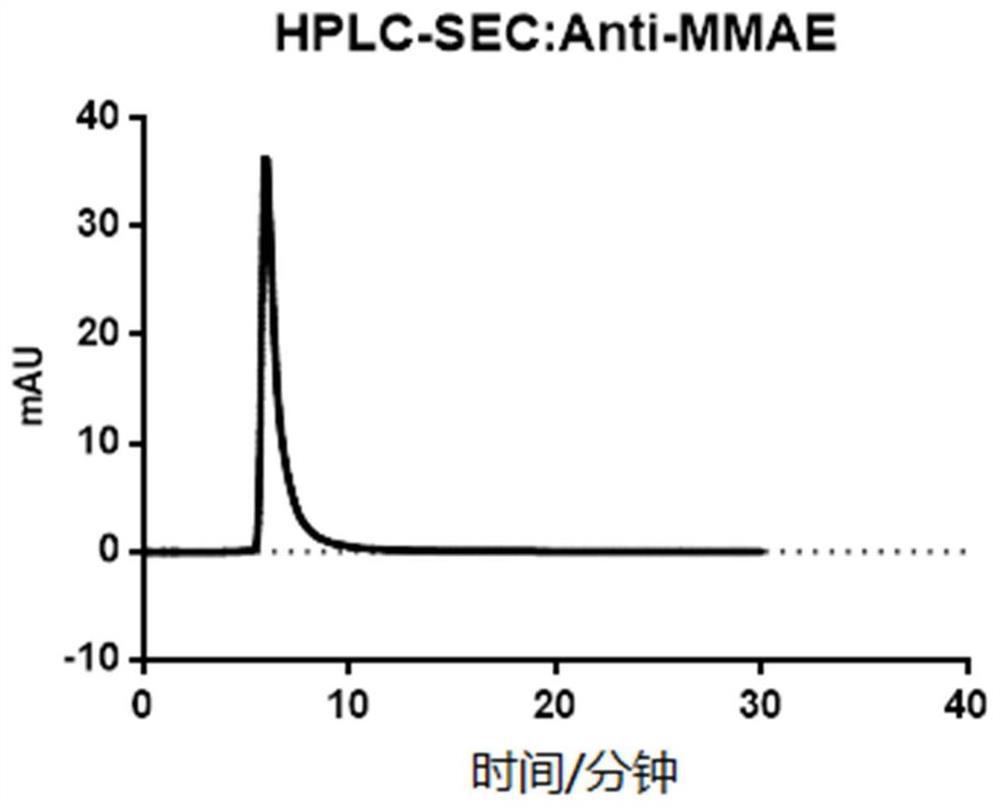
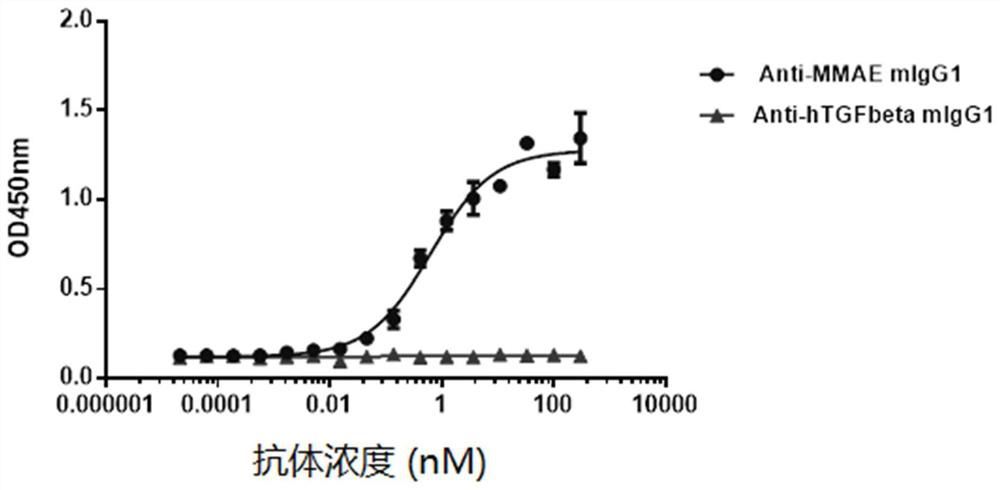
[0042] (5) 5-7 days after transfection, collect the cell supernatant, perform affinity purification with Protein G resin to obtain high-purity antibody protein, and replace the elution buffer with PBS with an ultrafiltration column. The amino acid sequence of the heavy chain variable re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com