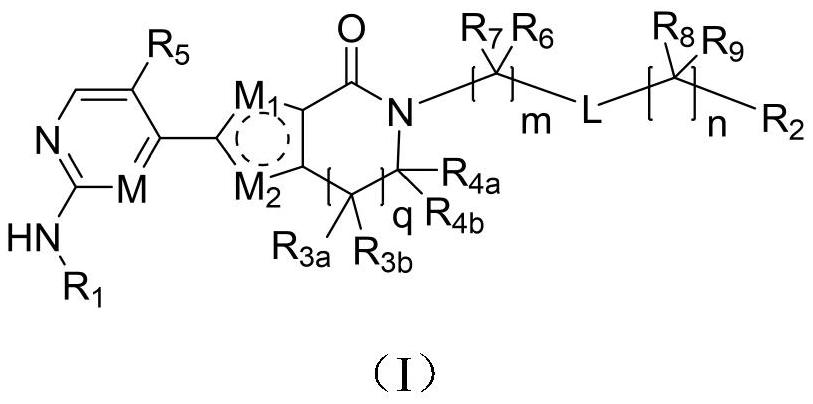
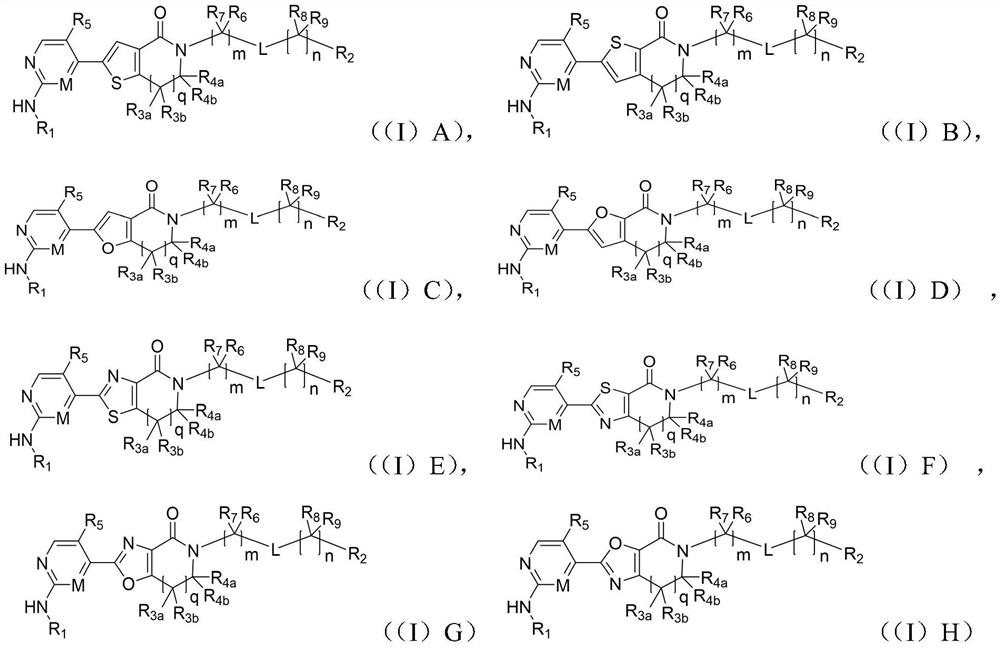
Aromatic heterocyclic lactam compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve problems such as low activity, single structure, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
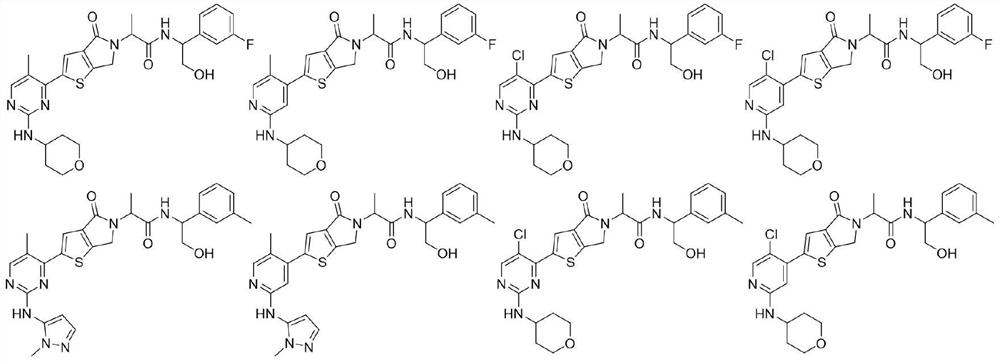
[0196] Example 1: (R)-N-((S)-1-(3-chlorophenyl)-2-hydroxyethyl)-2-(2-(2-((1-methyl-1H-pyridine Azol-5-yl)amino)pyridin-4-yl)-4-oxo-4,6-dihydro-5H-thien[2,3-c]pyrrol-5-yl)propionamide
[0197]
[0198] The first step: (4-bromopyridin-2-yl)(1-methyl-1H-pyrazol-5-yl) tert-butyl carbamate (150mg, 0.42mmol), pinacol borate (119mg, 0.47mmol), potassium acetate (123mg, 1.26mmol) and 1,1'-bisdiphenylphosphinoferrocenepalladium dichloride (Pd(dppf)Cl 2 ) (61mg, 0.084mmol) was dissolved in dioxane (10mL), nitrogen was bubbled for 10 minutes, and heated to 80°C for 8 hours under nitrogen protection. The reaction solution was cooled to room temperature, filtered, and concentrated under reduced pressure to obtain (1-methyl-1H-pyrazol-5-yl)(4-(4,4,5,5-tetramethyl-1,3,2- Dioxin-2-yl)pyridin-2-yl)tert-butyl carbamate (yellow oil) was directly used in the next reaction. LC-MS: ESI [M+H]+=401.2; 1 H-NMR(DMSO-d6,400MHz)δ8.34-8.35(m,1H),7.92(s,1H),7.88(s,1H),7.38(d,J=2.0Hz,1H),6.14(d , J=...
Embodiment 2
[0203] Example 2: (R)-N-((S)-1-(3-chlorophenyl)-2-hydroxyethyl)-2-(2-(5-methyl-2-((1-methyl Base-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)-4-oxo-4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propane Amide
[0204]
[0205] The first step: tert-butyl (R)-2-(4-oxo4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propionate (1.0g, 3.8 mmol), bispinacol borate (B 2 pin 2 ) (531 mg, 2.1 mmol), 4,4-di-tert-butylbipyridine (dtbpy) (20 mg, 0.076 mmol) and 1,5-cyclooctadiene methoxyiridium [Ir (cod) OMe] 2 ( 25mg, 0.038mmol) was dissolved in tetrahydrofuran (THF) (20mL), replaced with nitrogen for 10 minutes, and heated to 80°C under the protection of nitrogen to react overnight. The reaction solution was cooled to room temperature, filtered, and concentrated under reduced pressure to obtain (R)-2-(4-oxo-2-(4,4,5,5-tetramethyl-1,3,2-dioxin-2 -yl)-4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propanoic acid tert-butyl ester (1.4g, red oily substance), directly used in the next reaction. LC-MS:ESI[M+H] ...
Embodiment 3
[0210] Example 3: (2R)-N-(2-hydroxyl-1-(m-toluene)ethyl)-2-(2-(5-methyl-2-((1-methyl-1H-pyrazole -5-yl)amino)pyridin-4-yl)-4-oxo-4,6-dihydro-5H-thiophene[2,3-c]pyrrol-5-yl)propionamide
[0211] Example 3 was synthesized by the same method as Example 1, LC-MS: ESI (M+H) 530.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com