Application of bismuth sulfide catalyst with sulfur vacancies
A catalyst, the technology of bismuth sulfide, applied in the field of photocatalytic materials, can solve the problems that bismuth sulfide does not have good nitrogen fixation ability, does not have near-infrared nitrogen fixation performance, wastes energy, etc., achieves good photocatalytic nitrogen fixation performance, broadens the absorption range, increases The effect of high utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 1.94g of bismuth nitrate pentahydrate in 30mL of ethylene glycol solution at room temperature, and thoroughly ultrasonicate and stir for 20 minutes to obtain a clear solution. , fully ultrasonicated and stirred for 20 minutes until a clear solution was recorded as solution B; after solution B was added dropwise to solution A, stirring was continued for 30 minutes to obtain a black mixed solution. The mixed solution was put into a 50mL autoclave, and subjected to a constant temperature reaction at 180°C for 3h. After cooling to room temperature, the precipitate was centrifuged, washed with water and ethanol three times, and dried at 60°C to obtain the final product Bi 2 S 3 -3.
Embodiment 2
[0032] Dissolve 1.94g of bismuth nitrate pentahydrate in 30mL of ethylene glycol solution at room temperature, and thoroughly ultrasonicate and stir for 20 minutes to obtain a clear solution. , fully ultrasonicated and stirred for 20 minutes until a clear solution was recorded as solution B; after solution B was added dropwise to solution A, stirring was continued for 30 minutes to obtain a black mixed solution. The mixed solution was put into a 50mL autoclave, and subjected to a constant temperature reaction at 180°C for 1h. After cooling to room temperature, the precipitate was centrifuged, washed with water and ethanol three times, and dried at 60°C to obtain the final product Bi 2 S 3 -1.
Embodiment 3
[0034] Dissolve 1.94g of bismuth nitrate pentahydrate in 30mL of ethylene glycol solution at room temperature, and thoroughly ultrasonicate and stir for 20 minutes to obtain a clear solution. , fully ultrasonicated and stirred for 20 minutes until a clear solution was recorded as solution B; after solution B was added dropwise to solution A, stirring was continued for 30 minutes to obtain a black mixed solution. The mixed solution was put into a 50mL autoclave, and subjected to a constant temperature reaction at 180°C for 5h. After cooling to room temperature, the precipitate was centrifuged, washed with water and ethanol three times, and dried at 60°C to obtain the final product Bi 2 S 3 -5.
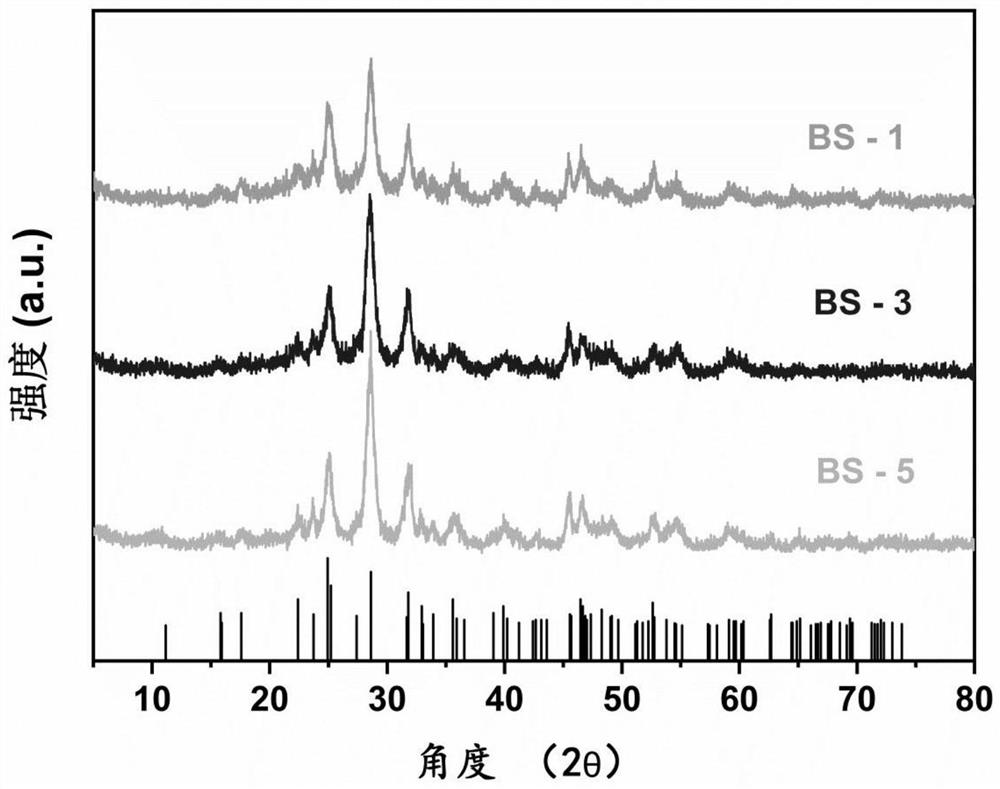
[0035] figure 1 The X-ray diffraction pattern of the sulfur-vacancy bismuth sulfide photocatalyst obtained in Examples 1-3 is shown. Compared with the PDF standard card, it is known that Bi 2 S 3 .
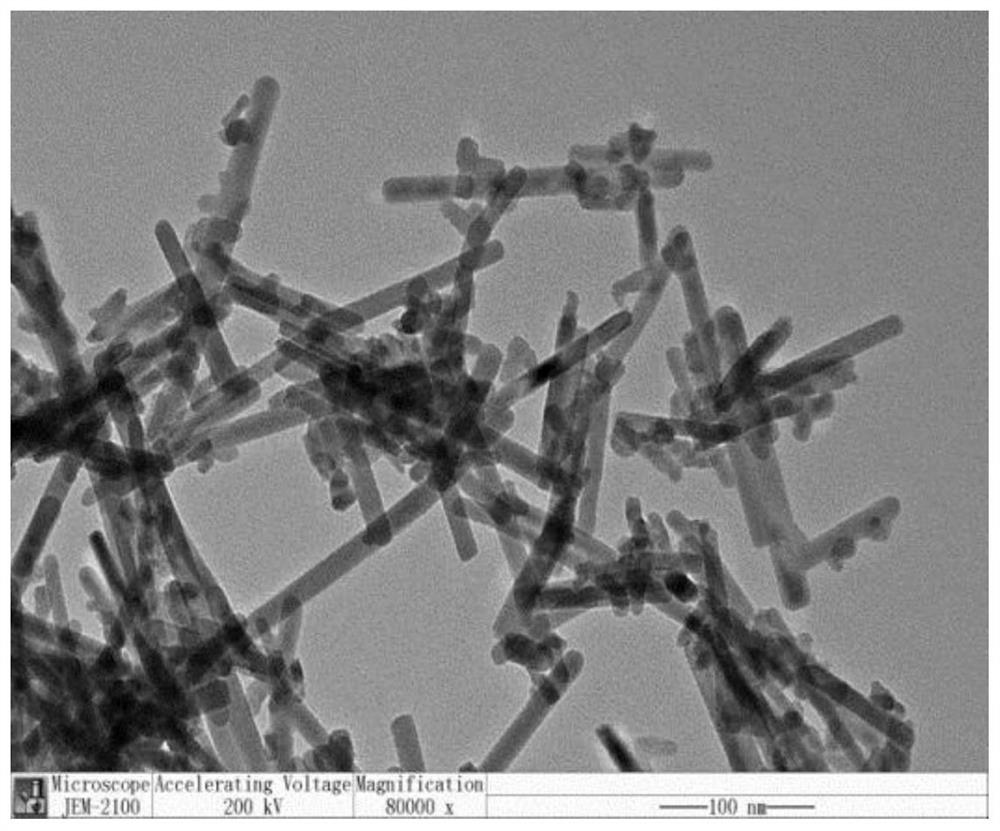
[0036] figure 2 The scanning electron microscope image of the sulfur-vacancy bis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com