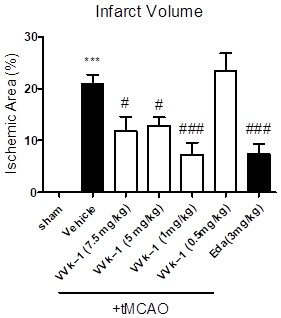
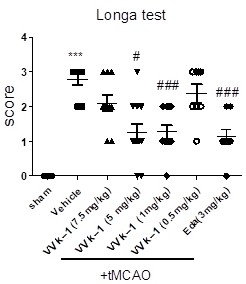
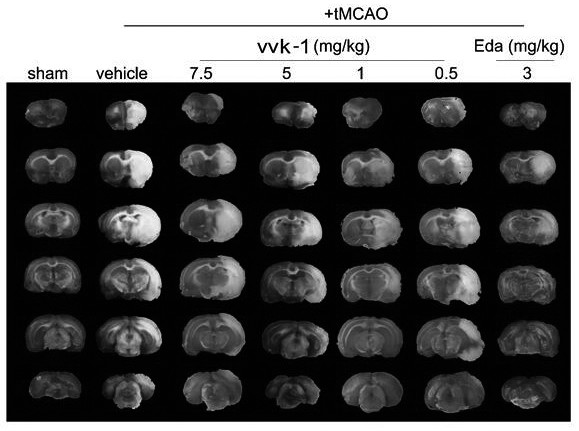
Proline borneol derivative as well as preparation method and application thereof in preparation of medicines for treating cardiovascular and cerebrovascular diseases
A technology for cardiovascular and cerebrovascular diseases and proline cinnol, which is applied in the field of medicine, can solve the problems of low bioavailability of stachydrine hydrochloride, high toxicity, restricting research and development, etc., so as to reduce the size of cerebral infarction and inhibit cytotoxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: N -methyl- L -The synthetic route of proline a is as follows:
[0032] ;
[0033] Will L - Dissolve proline (4.0 g, 34.8 mmol) in methanol (40 mL), add 40% formaldehyde aqueous solution (2.8 mL, 38.2 mmol) and 10% palladium carbon (1 g) in sequence, add hydrogen, and add hydrogen at room temperature Reacted for 24 hours, after the reaction was complete, filtered, and the mother liquor was concentrated and dried to obtain 4 g of white solids, with a yield of 89%, namely N -methyl- L - Proline (compound a). ESI-MS: (m / z, %) = 130 [M+H] + .
Embodiment 2
[0034] Example 2: The synthetic route of 1,7,7-trimethylbicyclo[2.2.1]hept-2-ylmethyl-L-prolylglycine (compound WK-1) is as follows:
[0035] ;
[0036] The described that embodiment 1 is made N -methyl- L - Proline (1.29 g, 10 mmol) was added to dichloromethane (20 mL), followed by addition of dicyclohexylcarbodiimide DCC (2.47 g, 12 mmol) and bartinol glycinate (2.1 g, 10 mmol), After reacting at room temperature (25°C) for 24 hours, the white solid was removed by filtration, and the mother liquor was concentrated to dryness to obtain 1.6 g of white solid with a yield of 50%, which was compound WK-1.
[0037] 1 H NMR (500 MHz, CD 3 OD) δ = 4.43 (t, J = 8.4 Hz, 1H), 4.13 (s, 2H),3.82-3.71 (m, 1H), 3.37-3.25 (m, 1H), 3.02 (s, 3H), 2.73-2.67 (m, 1H), 2.25- 2.18 (m, 2H), 2.17-2.06 (m, 1H), 1.96-1.65 (m, 7H), 1.02 (s, 3H), 0.98 (s,6H).
Embodiment 3
[0038] Example 3: The synthetic route of 1,7,7-trimethylbicyclo[2.2.1]hept-2-ylmethyl-L-propylcarbamate (compound WK-2) is as follows:
[0039] ;
[0040] The described that embodiment 1 is made N -methyl- L -Proline (1.29 g, 10 mmol) was added to dichloromethane (20 mL), followed by dicyclohexylcarbodiimide DCC (2.47 g, 12 mmol) and bartinol alanine (2.2 g, 10 mmol ), reacted at room temperature for 24 hours, filtered off the white solid, and concentrated the mother liquor to dryness to obtain 2.0 g of white solid with a yield of 60%, which was compound WK-2.
[0041] 1 H NMR (500 MHz, CD 3 OD) δ = 4.45 (t, J = 8.4 Hz, 1H), 4.12 (s, 1H),3.81-3.73 (m, 1H), 3.35-3.25 (m, 1H), 3.05 (s, 3H), 2.75-2.68 (m, 1H), 2.26- 2.18 (m, 2H), 2.19-2.07 (m, 1H), 1.97-1.64 (m, 7H), 1.42 (s, 3H), 1.05 (s,3H), 0.99 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com