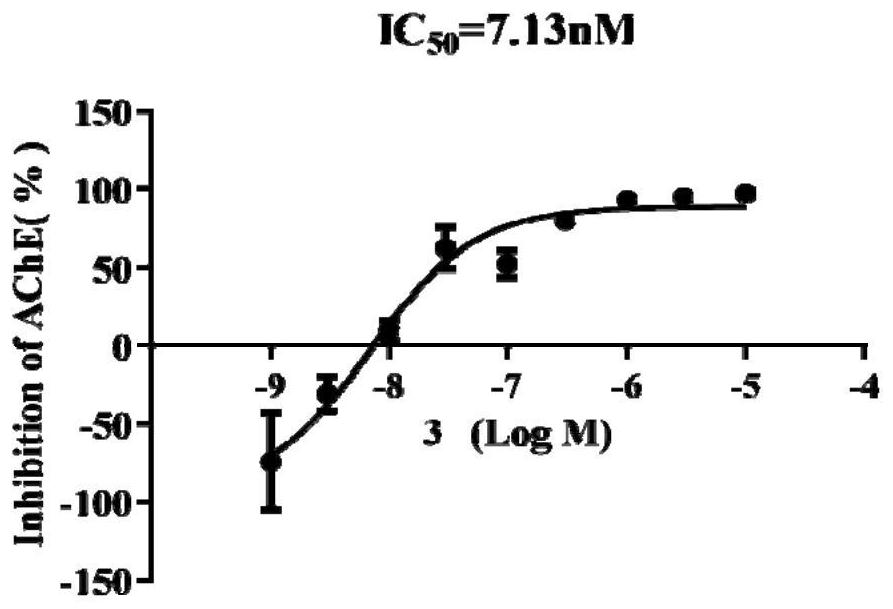
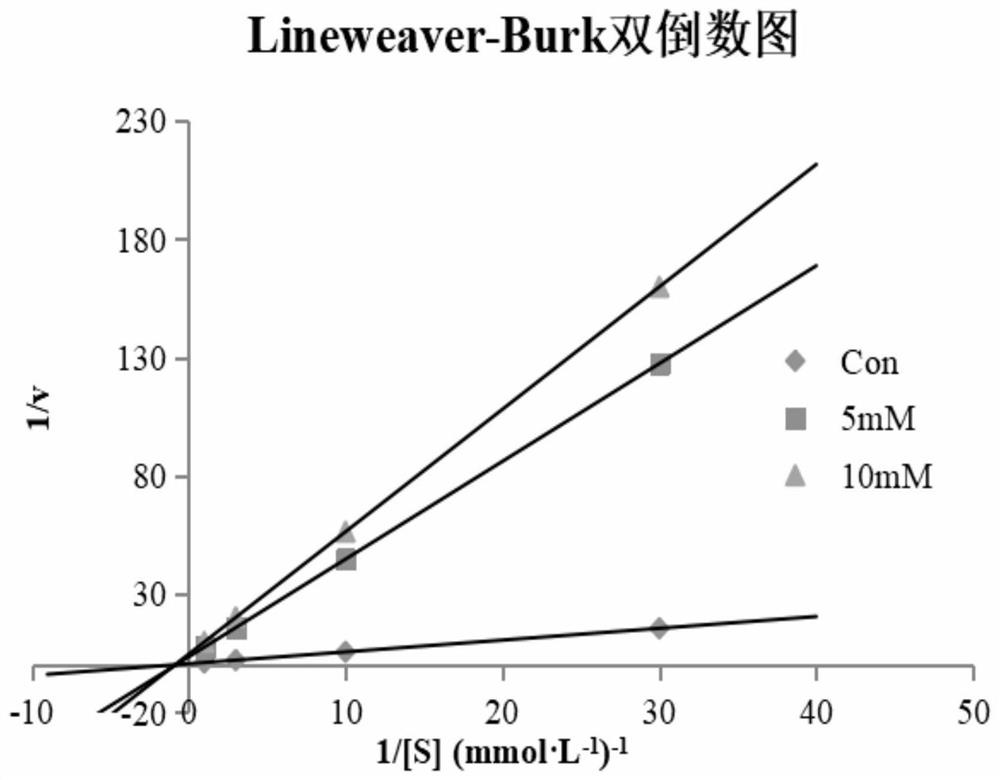
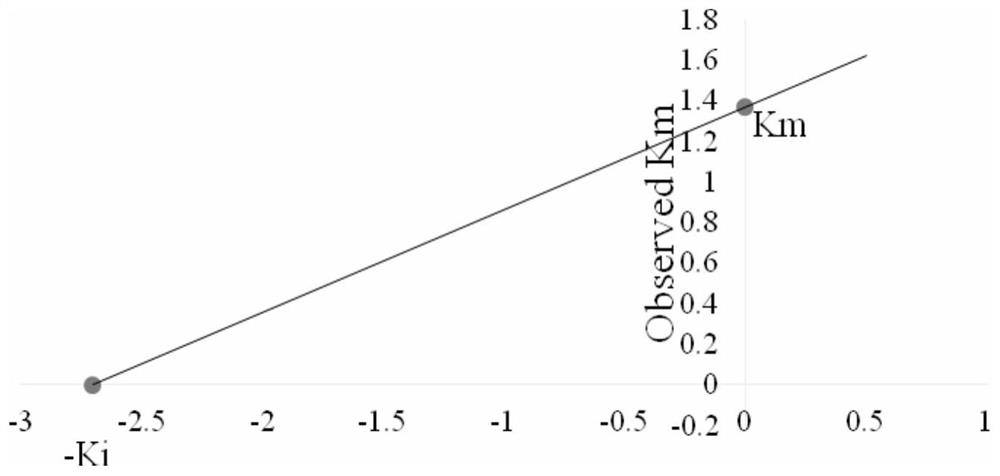
1, 2, 3, 4-tetrahydroacridine-9-amine compound as well as preparation method and application thereof
The technology of an amine compound and tetrahydroacridine, which is applied in the field of medicine, can solve the problems of inability to adapt to the pathological environment and single drug target, and achieve the effect of easy-to-obtain raw materials, simple preparation method, and weaken Aβ neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0048] A novel 1,2,3,4-tetrahydroacridin-9-amine compound, the compound is a compound with the structural formula shown in formula I and II, or a pharmaceutically Acceptable salts, esters or solvates,
[0049]
[0050] Among them, R 1 for H, CH 3 , OCH 3 , OCH 2 CH 3 , Cl, Br, CF 3 , NO 2 , NH 2 Or straight-chain alkanes with carbon number 1-5, R 2 , R 3 for H, n=1, 2, 3, 4 or 5, m=1, 2, 3, 4 or 5, the pharmaceutically acceptable salt, ester or solvate of the compound described in the above formula I and II, wherein the salt is an inorganic acid Salts or organic acid salts; inorganic acid salts are selected from the salts formed by any of the following inorganic acids: hydrochloric acid, sulfuric acid and phosphoric acid; organic acids are selected from the salts formed by any of the following organic acids: acetic acid, trifluoroacetic acid, propane Diacids, citric acid and terephthalic acid.
[0051] The novel 1,2,3,4-tetrahydroacridin-9-amine compounds r...
specific Embodiment 2
[0054] The specific embodiment above is a preparation method of a novel 1,2,3,4-tetrahydroacridin-9-amine compound, comprising the following steps:
[0055] (1) react the compound of formula III and sodium hydroxide in methanol aqueous solution to obtain the compound of formula IV; specifically, at 40°C, dissolve the compound of formula III and sodium hydroxide in an equal volume ratio of 1:1.3 molar ratio React in the mixed aqueous methanol solution for 6 hours to obtain the compound shown in formula IV; thus, it is beneficial to improve the reaction efficiency, reduce side reactions, and increase the yield;
[0056] (2) react the compound of formula IV and the compound of formula V in phosphorus oxychloride to obtain the compound of formula VI; specifically, at 0°C, add the compound shown in formula IV and the compound shown in formula V according to the molar ratio of 1:1.2 In phosphorus oxychloride, transfer to 110 DEG C and react for 6 hours to obtain the compound shown i...
Embodiment 1
[0064] Example 1: Compound 1 preparation of
[0065] 1. Preparation of 2-aminobenzoic acid
[0066] Ethyl 2-aminobenzoate (6.06 mmol) and sodium hydroxide (7.878 mmol) were stirred in methanol (5 mL) and water (5 mL), and the mixture was stirred at 40° C. for 6 hr. After the reaction was completed, methanol was removed by rotary evaporation, and the resulting solution was acidified to pH 3-4 with hydrochloric acid. The resulting suspension was stirred for 10 minutes, the precipitate was filtered and dried to obtain a white solid powder with a yield of 95%. The structural data of the compound was characterized as: 1 H NMR (600MHz, CDCl 3 ):7.69(d, J=7.8,1H),6.93(t,J=7.6,1H),6.45(d,J=8.1,1H),6.37(t,J=6.4,1H),3.17(s, 1H).
[0067] 2. Preparation of 9-chloro-1,2,3,4-tetrahydroacridine
[0068] The intermediate 2-aminobenzoic acid (3.6 mmol) and cyclohexanone (4.32 mmol) obtained in the previous step were added into phosphorus oxychloride (10 mL) at 0°C, and then refluxe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com